Article Text
Abstract
Background There is evidence of increasing use of the synthetic cathinone mephedrone (4-methylmethcathinone), particularly amongst clubbers. However, there have only two single case reports of mephedrone toxicity. The aim of this study is to report the pattern of clinical toxicity seen with mephedrone use.
Case Series We describe 15 patients who presented to our Emergency Department following self-reported mephedrone use. Significant clinical features seen included agitation in 53.3%, tachycardia in 40%, systolic hypertension in 20% and seizures in 20%. Twenty per cent required treatment with benzodiazepines, predominantly for management of agitation. All patients were discharged with no sequelae. Previous user reports have suggested that mephedrone use is associated with cool/blue peripheries; this was not seen in any of the patients in our series.
Conclusion The pattern of toxicity seen with mephedrone in this series is similar to that seen with 1-benzylpiperazine which has recently been classified under UK and EU misuse of drugs legislation. On the basis of this, together with a recent confirmed mephedrone related death in Sweden, we feel that appropriate assessments should be undertaken to determine the legal status of mephedrone.
- Mephedrone
- 4-methylmethcathinone
- 4-MMC
- cathinone
- toxicity
Statistics from Altmetric.com
The pattern of recreational drug use has changed over the last decade, particularly amongst those individuals who go clubbing.1 2 Particularly noticeable is the fall in the ‘recent’ (last month) reported use of MDMA (3,4-Methylenedioxymethamphetamine or ‘ecstasy’) amongst clubbers, from 79.3% in 1999 only 48.4% in 2009. This fall in the use of MDMA is postulated to have occurred due to decreasing purity and concentrations of MDMA on sale to users. A number of surveys have demonstrated an increasing use of synthetic cathinones, in particular mephedrone.2 3 Life-time use prevalence of mephedrone amongst a sample of 2200 clubbers was reported to be 41.7%, with 33.6% of those surveyed reporting use within the last month.2 Of those who had tried mephedrone, 14.5% reported that they were using it on an ‘at least weekly’ basis.
Mephedrone (4-methylmethcathinone) is a synthetic cathinone, related to the pharmacologically active alkaloid cathinone found in the leaves of the Khat plant.4 5 Mephedrone is a keto-amphetamine, which potentiates the release of pre-synaptic catecholamines, which explains their stimulant properties.4–8 The sale of mephedrone to users appears to be largely through the internet and high street ‘head shops’, in contrast to classical controlled recreational drugs such as MDMA.2 9 It is often marketed to users as either plant food or bath salts, which are sold ‘not for human consumption’, under acronyms including ‘Miaow miaow’, ‘meow meow’, ‘snow’ and ‘bubbles’.2 3
There have been reports in the lay media, on user websites and in the recent clubbers survey of unwanted effects associated with the use of mephedrone.2 10–13 Commonly reported unwanted effects include sweating, headache, palpitations, nausea, vomiting and cool/discoloured fingers. However, there has been only two individual case reports in the medical literature of confirmed mephedrone toxicity following recreational use.9 14 The aim of this study is to describe the pattern of toxicity seen in patients presenting with recreational drug toxicity following self-reported mephedrone use.
Methods
Detailed clinical and epidemiological data on all acute poisonings, including those that are recreational drug related, presenting to our large inner-city teaching hospital Emergency Department (ED) are prospectively collected on a purpose designed clinical toxicology database.15 This database was interrogated retrospectively to identify patients who had presented to our ED following the acute self-reported use of mephedrone between 1 January 2007 and the 31 December 2009 inclusive.
Data on the sex and age of the patient, co-ingested recreational drugs/ethanol, presenting physiological symptoms/signs (eg anxiety/agitation, seizures, vomiting) and presenting physiological parameters (heart rate, systolic blood pressure, temperature, Glasgow Coma Score (GCS) and pupil size) were extracted on the identified patients. Additionally, the length of stay was calculated and whether there were any complications related to the presentation/drugs ingested was recorded.
Results
Study population
There were no presentations to our ED with self-reported mephedrone use prior to 2009. There were a total of 15 presentations relating to mephedrone use in 2009, of whom 12 (80%) were male. The mean age (±SD) of the patients was 29.1±7.7 years; there was no significant difference between the mean age of the males (29.6±8.3 years) compared with the females (27.0±5.3 years; p=0.62).
Concomitantly used drugs
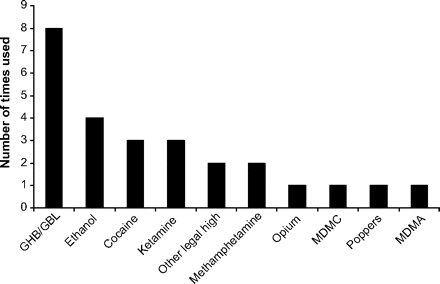
None of the presentations reported lone mephedrone use, and the mean ±SD concomitantly used drugs/ethanol was 1.7±1.0 (range, 1–4). Four patients had also used ethanol at the same time as the mephedrone. The remaining patients had concomitantly used ≥1 other recreational drug and the frequency of other drugs concomitantly used is shown in figure 1. Interestingly, there were two other ‘legal highs’ (neo-doves and neo-blues) used, although it is not certain what drug(s) these particular branded legal highs contained.
Self-reported concomitantly used drugs in patients presenting to the ED following the use of mephedrone.
Physiological parameters and symptoms on presentation
The baseline physiological parameters on presentation to the ED are summarised in table 1. 20% had clinically significant hypertension (systolic blood pressure ≥160 mmHg) and 40% of had a tachycardia (heart rate of ≥100 bpm). The majority of patients (60%) had a GCS of 15 on presentation to the ED; of the four who had a GCS of ≤8, all had concomitantly used a CNS depressant (GHB/GBL in three presentations and opium in one presentation).
Baseline physiological parameters in patients presenting to the ED following the use of mephedrone
The proportion of patients with clinical symptoms/signs reported by other authors with mephedrone toxicity on presentation to the ED is shown in table 2. The most common clinical symptom/sign on presentation was agitation (53.3% of patients). Additionally, there were three (20%) who had a self-limiting pre-hospital seizure following the use of mephedrone. Interestingly, none of the patients who presented following the use of mephedrone had any skin discolouration or cool/cold peripheries which have previously been reported in user surveys.2
The proportion of patients with clinical symptoms/signs previously associated with mephedrone toxicity on presentation to the ED
Clinical management and outcome
The majority of patients (11; 73.3%) required either only a period of observation prior to discharge and/or symptom control (eg anti-emetics, intravenous fluids). Three (20%) patients required the use of benzodiazepines (oral or IV) for the management of agitation on presentation to the hospital. The final patient required endotracheal intubation for airway protection; they had evidence of significant GHB toxicity on presentation.
Eleven (73.3%) of patients were discharged either directly from the ED or after a short period of observation on the short stay ward. Of the four patients who were admitted to hospital, three were admitted for observation/management on a general internal medicine ward and one (6.7% of all presentations) required admission to an intensive care unit. All patients survived to discharge from hospital with no long-term sequalae on discharge. The overall mean length of stay following presentation to hospital, after exclusion of one patient who developed aspiration pneumonia secondary to opium toxicity and the patient admitted to ICU for GHB toxicity, was 8.4±8.3 (range, 1.2–28.0) h.
Discussion
We found that prior to the beginning of 2009, there were no presentations related to use of mephedrone to our Emergency Department. During 2009, 15 patients presented to the Emergency Department with recreational drug toxicity and self-reported use of mephedrone. The most common clinical feature seen on presentation was agitation present in over half of the patients, and over a third of these required treatment with benzodiazepines to control the agitation. Other clinically significant issues seen on presentation included tachycardia, hypertension and seizures.
A recent recreational drug use user survey has reported high prevalence of unwanted effects after the use of mephedrone, such as sweating (67%), headache (51%), palpitations (43%), and nausea (27%).2 In our study, the reporting of these symptoms was significantly lower than that in the user survey. It is possible that some of the symptoms described in the user survey were milder, and therefore did not require medical attention at the time. In particular, the user survey reported that 15% of individuals described ‘cold or blue fingers’ following use, which has also been reported by users in online discussion forums.2 16 We did not see this phenomenon in our patients, or in our previously reported case.14
Recently there have been reports in the lay media of deaths thought to be associated with the use of mephedrone, although confirmatory analytical findings are not yet available.17 Of the two confirmed mephedrone associated toxicity case reports, one patient had sympathomimetic clinical features that were managed with supportive care and benzodiazepines.14 The other case report was a patient after an out of hospital cardio-respiratory arrest related to use of mephedrone.9 Postmortem blood and urine toxicological analysis confirmed the presence of mephedrone, no other drugs were detected, and it is likely that this is the first confirmed fatality related directly to mephedrone.
This pattern of toxicity seen in our study is similar to the pattern of toxicity associated with the use of 1-benzylpiperazine.18 19 On the basis of the toxicity associated with 1-benzylpiperazine use, it underwent a ‘risk assessment’ by the European Monitoring Centre for Drugs and Drugs Addiction (EMCDDA) in 2007, although at the time of this there had been no confirmed fatalities related to the use of 1-benzylpiperazine. Following the EMCDDA risk assessment, 1-benzylpiperazine was controlled both at European level and under the UK Misuse of Drugs Act (1971) in 2009.20 21 We feel that there should be similar consideration for an assessment of mephedrone and its current ‘legal status’, particularly as there is a confirmed fatality associated with mephedrone use.
References
Footnotes
Competing interests DW and PD have acted as scientific advisors to the UK Advisory Council on the Misuse of Drugs (ACMD) and the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA).
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Primary survey