INTRODUCTION
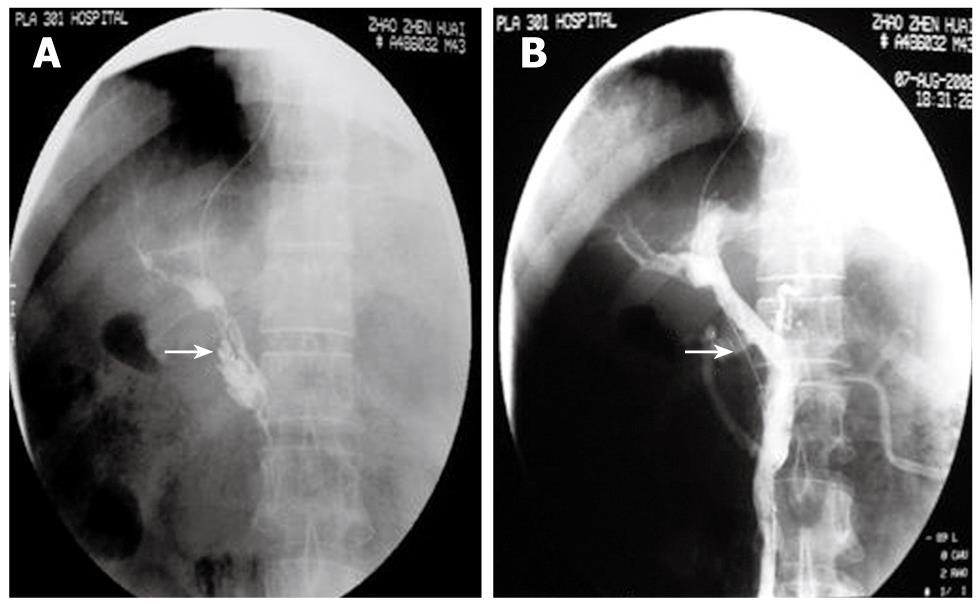
Figure 1 The figure shows a male, 43-year-old patient, 2 mo after splenectomy, with abdominal fullness for 6 d.
He had thrombolysis through a transjugular intrahepatic portosystemic shunt (TIPS). A: Direct PV-SMV angiography showed extensive thrombosis in the PV and SMV. Contrast agent remained. No lateral branch angiogenesis (arrow); B: Mash and suck thrombi with intermittent injection of urokinase and heparin sodium. Repeated angiography 30 min after thrombolysis showed blood reperfusion in the PV-SMV and normal PV-SMV branches (arrow). Symptoms disappeared.
Figure 2 The figure shows a female, 22 year old patient who had abdominal pain for 17 d.
A: A CT scan showed a high density of superior mesenteric vein thrombosis (characteristic of subacute thrombosis) (arrow); B: A Cobra catheter was inserted via the femoral artery into the superior mesenteric artery. Indirect angiography showed extensive thrombosis in the PV-SMV. Angiographic contrast agent remained (arrow); C: Indwelling catheters for 8 d. Angiography showed partial blood reperfusion in the PV-SMV. PV branches in the liver were intact (arrow). Symptoms were relieved.
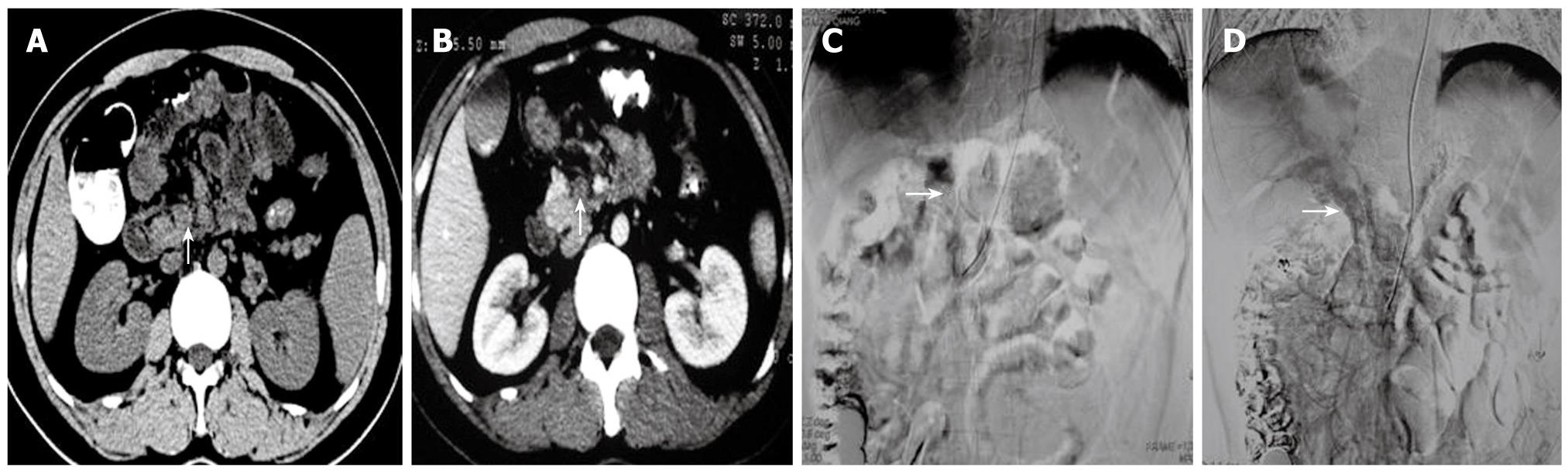
Figure 3 A 24 year old male with abdominal pain for 8 d.
A: The CT scan showed a low density SMV thrombosis (arrow); B: An enhanced CT scan still showed a low density of thrombosis (arrow). This fulfilled the symptoms and signs of acute thrombosis; C: An extended Cobra catheter was introduced into the superior mesenteric artery via the radial artery. The indirect angiography shows extensive thrombosis in the PV-SMV without lateral branch angiogenesis (arrow); D: Indwelling catheters for 10 d. The indirect angiography showed that the lateral vessels of the PV-SMV had significantly increased (arrow). Symptoms were relieved.
With the development of imaging technology, the rate of diagnosis of portal and superior mesenteric vein thrombosis has gradually increased[1]. When thrombosis occurs only in the portal vein, clinical symptoms such as abdominal pain, nausea, anorexia, and weight loss are usually not serious. When thrombosis involves the superior mesenteric vein, patients may have cramps in the upper middle or lower middle abdomen, nausea, anorexia, vomiting, diarrhea, and/or bloody stool[2-4]. Conservative medical treatment is often unsatisfactory. Surgical treatment is accompanied by more tissue damage, more complications, and a high recurrence rate. Therefore, it is seldom used as a standard treatment unless intestinal necrosis occurs. Recently, interventional and minimally invasive techniques were introduced to treat portal vein and superior mesenteric vein thrombosis (PV-SMV) thrombosis. The results have been excellent[5,6]. Herein we summarize the interventional treatment of 46 patients with symptomatic acute-subacute PV-SMV thrombosis. Our goal was to describe how to choose a specific interventional treatment, and how to enhance the effect of the treatment and reduce complications.
MATERIALS AND METHODS
General information
We reviewed the case histories of 46 patients including 30 males and 16 females, with an average age of 48 years (range: 17-68 years). All patients had different degrees of abdominal pain, fullness, and anorexia. Other symptoms and signs included diarrhea (25 cases), vomiting (11 cases), and ascites (8 cases). There was no obvious abdominal rigidity and rebound tenderness. Causes of the disease were clearly defined in 42 patients, including 18 who had splenectomies within one year, 8 who had a recent history of pancreatitis, 14 who had liver cirrhosis with portal hypertension (6 of them accompanied by esophageal and gastric varices), 1 who had the condition secondary to duodenal surgical repair for traumatic injury within the last 24 h, and 1 who had appendicitis within the last month.
The period of the disease included 28 acute cases (thrombosis occurred within 1 wk of onset) and 18 sub-acute cases (1-3 wk from onset). The number of platelets was higher (500-980 × 109/L) than standard scores in 28 patients. The number of white cells was higher (12-20 × 109/L) than normal standards in 17 patients. Red cell number and hemoglobin levels were in the normal range. Liver function tests showed 42 with Child A, and 4 with Child B. Renal function and plasma electrolytes were in the normal range. All patients were clearly diagnosed as having PV-SMV thrombosis by Doppler ultrasound scan, computed tomography (CT) and magnetic resonance imaging (MRI). They were fast and gastrointestinal decompressed.
Interventional treatment
Direct PV-SMV thrombolysis: Twenty four patients were seen within 1 wk of the onset of disease and diagnosed as having acute PV-SMV thrombosis without obvious lateral branch angiogenesis by imaging. Eight patients were seen between 1 and 2 wk from the onset of disease and diagnosed as subacute PV-SMV thrombosis without obvious lateral branch angiogenesis by imaging.
Thrombolysis through transjugular intrahepatic portosystemic shunts (TIPS): In 26 patients, a 10F long sheath (RUPUS 100 system, COOK Corp, USA) was used to puncture the right internal jugular vein according to Seldinger’s method and then advanced into the hepatic vein via the superior vena cava, right atrium, and inferior vena cava. A shell-type needle was introduced into the hepatic vein through the sheath and it was advanced into the right branch of the PV under X-ray guidance. When PV branches were filled with thrombi and blood flow was interrupted, it was not possible to determine whether PV was punctured or not using the aspirate puncture-cannula. Under such conditions, the contrast should be injected slowly via the puncture-cannula while the puncture-cannula is withdrawn gradually. When the puncture was successful, the contrast remained in the PV branches and the shape of vessels could be seen clearly. An ultra-smooth guide wire was easily introduced into the PV-SMV system. Once it was in the right branch of the PV, a 4F Cobra Catheter (Cordis Corp. USA) or an inferior vena cava angiographic catheter (COOK Corp, USA) was advanced to the distal SMV for direct PV-SMV angiography.
Before thrombolysis, 30-50 mg sodium heparin was injected intravenously into patients without any contraindications. An 8F large thin-walled introducer catheter (90 cm Cordis Corp., USA, or a 100 cm Boston Scientific Corp. USA) was used to aspirate the thrombi and a 4F pigtail catheter (Cordis Corp. USA) was used for mashing the thrombi. A 4F inferior vena cava catheter with multiple lateral holes (COOK Corp. USA) was inserted into the PV-SMV and used to deliver sodium heparin and urokinase (average doses of 0.8 MIU, 0.5-1.5 MIU) intermittently for local thrombolysis. After most of the thrombi in the PV-SMV had been removed, the catheter was maintained for continuous thrombolysis with urokinase (0.5-1.5 MIU/d) and sodium heparin (30-200 mg/d) for 3-13 d. Clopidogrel (75-150 mg/d) or enteric-coated aspirin (100-150 mg/d) were administered to patients with platelet counts greater than 300 × 109/L. During treatment, thrombin time (TT) and activated partial thromboplastin time (APTT) were monitored to maintain TT at 1.5-2.5 times the normal range, and APTT at 2-2.5 times the normal range. In the meantime, levels of D-dimer were monitored[7]. The duration of indwelling catheters, which depended on the improvement of symptoms and thrombolysis, usually lasted less than 2 wk. After the catheter was withdrawn, intravenous sodium heparin was continued for 2 additional weeks, and then changed to oral warfarin sodium (for not less than 1 year). Treatment with warfarin and heparin should overlap for 3 d. After being discharged from the hospital, patients were monitored with standard blood tests and we maintained the international normalized ratio (INR) at 2.0-3.0. Abdominal ultrasound scans were repeated every 1-3 mo and CT or MRI scans as necessary.
Thrombolysis through percutaneous transhepatic portal vein cannulation: In 6 patients, a percutaneous transhepatic puncture was performed with a 22-gauge Chiba needle (COOK Corp, USA) into the right branch of the PV, at the midline through the right armpit. Then an 8F artery sheath (Cordis Corp. USA) was introduced into the PV for thrombolysis. The following thrombolysis procedure was the same as the TIPS method. After treatment, the punctured tract was broken by a gelatin sponge and a steel ring (COOK Corp, USA) was used to prevent secondary liver bleeding.
Indirect PV-SMV thrombolysis
Patients who received indirect PV-SMV thrombolysis included: (a) 4 with acute PV-SMV thrombosis diagnosed by imaging tests within one week of the onset of the disease and who refused direct PV-SMV thrombolysis, (b) 4 with subacute PV-SMV thrombosis diagnosed within 1-2 wk, including 3 who refused and 1 who failed direct PV-SMV thrombolysis, (c) 6 with subacute PV-SMV thrombosis diagnosed within 2-3 wk, including 3 showing lateral branch angiogenesis around the PV-SMV system in the CT scan, suggesting a long duration of the disease.
Thrombolysis through the femoral artery to the superior mesenteric artery cannulation
In 10 patients, a 4F artery sheath (Terumo Corp. Japan) was used to puncture the right femoral artery according to a modification of Seldinger’s technique, and then a 4F Cobra Catheter was inserted to perform celiac artery, superior mesenteric artery and indirect PV-SMV angiography. When angiography was completed, 3-6 holes were drilled at the front of the Cobra Catheter with a microdriller. An intensive dose of urokinase (0.2 MIU) was administered into the superior mesenteric artery through the catheter. Afterward, the catheter remained in the superior mesenteric artery in order to continue the thrombolysis with urokinase (0.75-1.5 MIU/d) and sodium heparin (30-200 mg/d) for 3-11 d. All treatments during and after indwelling catheters and review were the same as those performed in the TIPS method.
Thrombolysis through the radial artery to superior mesenteric artery catheterization
The left radial artery in 4 patients was punctured with a radial puncture system (COOK Corp, USA) using a modified Seldinger’s method. A 5F artery sheath (COOK Corp, USA) was inserted and then an extended (120 cm) 5F Cobra Catheter (Terumo Corp. Japan) was introduced for celiac artery, superior mesenteric artery and indirect PV-SMV angiography. All other procedures were the same as those through the femoral artery to the superior mesenteric artery cannulation thrombolysis methods.
RESULTS
Direct PV-SMV thrombolysis was performed in 32 patients, taking 80-180 min each. In 22 of the 32 patients, direct PV-SMV angiography showed extensive thrombosis in the PV and SMV with contrast agent remaining in the vessels and without lateral branch angiogenesis (Figures 1A, 2B, 3C). Ten patients had thrombosis mainly in the PV, SMV and splenic vein. After thrombolysis, repeated angiography showed normal PV-SMV systems in 26 patients (Figure 1B) while thrombosis partially remained in the PV-SMV in 6 patients. Symptoms such as abdominal pain, fullness, and diarrhea disappeared or were significantly relieved. Hospital stays lasted from 2 wk to 2 mo. One patient whose thrombosis was secondary to duodenal surgical repair had PV-SMV reperfusion and symptomatic improvement but died because of an abdominal abscess and multiple organ failure. In 3 patients with interventional treatment, thrombosis recurred (at 1, 3 and 4 mo, respectively) after the treatment. For these 3 patients, indirect PV-SMV thrombolysis was performed again and repeated angiography showed increased lateral branch angiogenesis in the PV-SMV branches. Their symptoms were improved. All other patients had good PV-SMV circulation without abnormal symptoms. Ultrasound and CT scans did not show signs of recurrence.
In 14 patients treated with indirect PV-SMV thrombolysis, one patient with acute thrombosis and another one with subacute thrombosis had partial recovery of PV-SMV flow, partial thrombolysis, and complete symptomatic relief (Figure 2C). Eleven patients had intact thrombosis in the PV-SMV and obvious lateral branch angiogenesis. Their symptoms were dramatically improved with only slight abdominal pain and fullness, and less diarrhea remaining (Figure 3D). One patient with acute thrombosis had no improvement in symptoms 3 d after continuous thrombolysis and developed exudation around the remaining sheath. Ileum segmental necrosis was found on laparotomy 2 d after the catheter was withdrawn. Bowel resection was performed and treatment was continued with an anticoagulant treatment. Six patients with esophageal-gastric varices had obvious improvement confirmed by gastroscope examination after treatment. During follow-up, one patient had upper gastrointestinal bleeding 22 mo after the interventional treatment and was treated with endoscopic sclerotherapy (EIS). Other patients did not develop symptoms of PV-SMV thrombosis again.
DISCUSSION
PV-SMV thrombosis has a concealed onset without any specific symptoms and signs. The diagnosis is therefore, easily delayed. If the following conditions occur, the possibility of PV-SMV thrombosis should be considered in order to achieve early diagnosis and treatment. (1) unexplained abdominal pain, abdominal distension, especially with nausea, vomiting, and bloody stool; (2) intractable ascites; (3) unexplained bloody ascites; (4) unexplained portal hypertension; (5) unexplained upper gastrointestinal bleeding or progressive spleen enlargement without apparent splenic hyperfunction; (6) unexplained paralytic intestinal obstruction, necrosis or peritonitis, etc[8,9]. The diagnosis of PV-SMV thrombosis relies on imaging. Color Doppler ultrasound is very simple, noninvasive, and has a high negative predictive value. It should be chosen first. If a positive result is found, CT or MRI scans should be considered for further investigation. Accurate judgment of the PV-SMV thrombosis duration - acute, subacute, or chronic - is extremely important for disease management[10]. According to our experience, thrombosis shown in a CT scan has a low density during the acute period (within 1 wk of the onset of the disease) (Figure 3A). It has a high density during the subacute period (1-3 wk after disease onset) with a CT value of 5-15 HU which is higher than values for the abdominal aorta and inferior vena cava (the so-called CT scan mesenteric vein angiographic phenomenon, an important piece of diagnostic evidence). It has a low density during the chronic period (> 3 wk) and is accompanied by lateral branch angiogenesis. The density of the thrombosis is not increased with contrast (Figure 3B)[11]. In the MRI scan, PV-SMV thrombosis is shown as a T1WI low signal and a T2WI high signal during the acute period. During the sub-acute period, both T1WI and T2WI signals are high. During the chronic period, T1WI gives mixed signals and T2WI gives low signals. After Gd-DTPA injection, the signal for thrombosis is not increased[12].
Traditional PV-SMV thrombolysis treatment includes conservative internal treatment and surgical treatment. Medical treatments include thrombolysis and anticoagulation, and others, which can improve symptoms in some patients. But conservative treatment cannot directly remove the obstruction due to the thrombosis. Therefore, its efficiency is very limited and the mortality rate due to gastric bleeding is high[13]. The application of surgical treatment is limited by tissue damage and additional complications[14-17]. With the development of interventional radiology, minimally invasive technology has become one of the predominant means of treating acute-subacute PV-SMV thrombosis without obvious intestinal necrosis, perforation, and peritonitis[18-26]. The method includes direct and indirect PV-SMV thrombolysis[27]. In our study, 46 patients with acute-subacute PV-SMV thrombosis were treated interventionally. The thrombolysis was effective without severe complication.
Our study showed that the effect of direct thrombolysis is better than indirect treatment. Injecting thrombolytic agents directly into a PV-SMV thrombus can dramatically increase the effect of thrombolysis, reduce the dose of thrombolytic agent, and reduce the complication of bleeding. Using a mechanical method such as aspirating and mashing to eliminate the thrombi, balloon expanding, stent implantation, etc. can result in reperfusion within a short time and recover blood circulation[28]. However, when the disease duration is too long (> 2 wk) or angiography shows some lateral branch angiogenesis around the main vessels, there is no indication for TIPS and surgical treatment; or, if the treatment via TIPS fails, indirect PV-SMV thrombolysis is still an option[24]. Evaluation of the efficacy of indirect PV-SMV thrombolysis should rely not only on reperfusion of the main vessels. The improvement in clinical symptoms and lateral branch angiogenesis with treatment are also important indications of efficacy. We found that indirect PV-SMV thrombolysis is simple and easy. When direct thrombolysis is difficult to perform, indirect thrombolysis can dissolve some thrombi, promote lateral branch angiogenesis and relieve symptoms for acute-subacute patients. With thrombolysis through the radial artery, one can achieve superior mesenteric artery indwelling catheters. Patients with such indwelling catheters do not need to rest in bed. Complications such as bleeding at the puncture point and infection are significantly reduced. Therefore, the procedure does not introduce any inconveniences into the patient’s daily life and is easily accepted by patients.
Regarding the choice of pathways for direct PV-SMV thrombolysis, the way through TIPS does not pass through the intraperitoneal cavity. Hence, it is suitable for patients with existing ascites, coagulative dysfunction and catheters that have been indwelling for a long time[22]. It has a specific advantage in prevention of bleeding, not only avoiding hepatic surface injury, but also reducing bleeding due to thrombosis being aspirated through the big vessel sheath (> 7F) because the cannula is inside the liver. Moreover, cannulation through TIPS can also divert portal blood flow and effectively relieve portal hypertension[29,30]. Therefore, it is very suitable for patients requiring a portosystemic shunt for portal hypertension and embolism for esophageal-gastric varices. The disadvantages of TIPS are its complexity and difficulties in performing it[31]. The procedure for percutaneous transhepatic portal vein cannulation is simpler, easier and cheaper than TIPS[25,32]. It is suitable for patients without ascites and coagulative dysfunction[33]. Recently, this procedure has been improved in several ways. Ultrasound was used to guide a fine needle to the puncture. A steel coil and a gelatin sponge were used to fill the puncture channel to reduce intraperitoneal haemorrhage[25,34]. This method can be an alternative for cases of unsuccessful TIPS or cases that are unsuitable for TIPS but that require direct PV-SMV thrombolysis.
During the time that the catheter is indwelling, determining safe and effective doses of urokinase and heparin is fundamental for the success of the treatment. Our study suggests doses for urokinase (0.5-1.5 MIU/d) and heparin sodium (30-200 mg/d). Urokinase should be rapidly injected through the catheter within half an hour, twice a day. Heparin sodium can be admitted via peripheral veins when urokinase treatment is performed via the catheter. It must be administered via the catheter to the thrombosis in the time gap between urokinase treatments. Patients with PV-SMV thrombosis usually have complications of chronic hepatic disease, clotting factor insufficiency, and generally low coagulation conditions but high coagulation conditions in the portal vein. The direct injection of urokinase and heparin into the PV is more effective. Clopidogrel or enterically-coated aspirin are administered for patients with platelet counts more than 300 × 109/L. In our study, one patient had a platelet count of 600-970 × 109/L while they had indwelling catheters after splenectomy and was treated with hydroxycarbamide to inhibit platelet formation.
Urokinase is a plasminogen activator. Its half-life is short (15-20 min). Quickly administering urokinase can instantly lead to its penetration into the thrombosis and cause thrombolysis. In our study, urokinase was injected within 30 min. If the injection is too slow, the effect of thrombolysis becomes weak. Urokinase can also cause degeneration of some clotting factors such as fibrinogen (FIB). FIB is an important clotting factor and a reflective index for activation of the fibrinolysis system. It is also a fundamental factor for plasma viscosity and platelet aggregation. The elevation of plasma FIB can promote blood coagulation and form clots. Because the half-life of urokinase is very short, FIB will increase after 24 h of urokinase administration and return to previous levels after 48 h. If it is not combined with other anticoagulation treatments, it will increase the incidence of vascular thrombosis obstruction after thrombolysis. Therefore, anticoagulation treatment during thrombolysis is necessary.
Heparin sodium affects blood clotting by inhibiting synthesis of fibrous protein factors and the extension of existing clots within the blood. In our study, all patients had anticoagulation treatment with a combination of urokinase and heparin sodium. The prevalence of hemorrhage is high (23%-28%)[21,35-38]. Therefore, an emergency treatment including ECG monitoring and blood pressure control for bleeding must be prepared. All indexes for coagulation and anticoagulation should be monitored. Presently, most researchers suggest that maintaining TT at 1.5-2.5 times the normal range and APTT at 2-2.5 times the normal range can not only achieve the best treatment efficacy, but also avoid severe bleeding. In our study, only three patients had exudation around the catheter sheath. Their symptoms were relieved with a compression bandage without any severe organic bleeding. This is related to understanding the time window of urokinase and heparin, and monitoring the dose for both medications according to all factors involved in coagulation and anticoagulation.
COMMENTS
Background
With the development of imaging technology, the rate of diagnosis of portal and superior mesenteric vein thrombosis has gradually increased. Thrombosis of portal vein and superior mesenteric vein thrombosis (PV-SMV) is a severe disease. The consequences of these thromboses can be severe, including mesenteric ischemia and variceal bleeding, with high mortality rate. There are no uniform protocols for the effective treatment of PV-SMV thrombosis. Conservative medical treatment is often unsatisfactory. Surgical treatment is accompanied by more tissue damage, more complications, and a high recurrence rate.
Research frontiers
The treatment of symptomatic acute thrombosis of the PV and SMV is controversial due to unsatisfactory results obtained in some cases with medical treatment, as well as the difficulty in performing surgical procedures in some cases. Recently, interventional and minimally invasive techniques were introduced to treat PV-SMV thrombosis.
Innovations and breakthroughs
The authors summarize the interventional treatment of 46 patients with symptomatic acute-subacute PV-SMV thrombosis, which demonstrated the feasibility of this method in the management of this challenging illness. Compared to conservative medical and surgical treatment, interventional treatment has the least tissue damage, complications, invasive and a high success rate.
Applications
Interventional endovascular thrombectomy with direct or indirect thrombolysis can offer a non-surgical alternative for the treatment of symptomatic acute-subacute PV-SMV thrombosis. This technique can be performed in patients who do not present with bowel ischemia and infarction, or who are not at risk for bleeding, and have persistent symptoms or worsening of symptoms despite anticoagulation.
Terminology
PV-SMV: Portal vein and superior mesenteric vein thrombosis. TIPS: Transjugular intrahepatic portosystemic shunts. TT: Thrombin time. APTT: Activated partial thromboplastin time. INR: International normalized ratio.
Peer review
This study summarizes the interventional treatment of 46 patients with symptomatic acute-subacute PV-SMV thrombosis.