- 1Department of Oncology and Hematoncology, Interdisciplinary Research Center on Decision Making Processes, University of Milan, Milan, Italy
- 2Applied Research Division for Cognitive and Psychological Science, IEO Istituto Europeo di Oncologia, Milan, Italy
Background: Genetic testing is performed for different purposes, such as identifying carriers, predicting a disease onset in presymptomatic individuals or confirming a diagnosis. However, these tests may have notable psychological effects, such as generating anxiety and depression. These effects may depend on people's perception of risk, severity, and controllability of the disease; and the availability of treatments. To date, there are no reports that analyze these factors specifically, and their role in influencing genetic test users' experience.
Methods: We performed a systematic review of the psychological implication of undergoing genetic testing for cardiovascular, neurodegenerative and cancer diseases. Articles were searched on PubMed, Google Scholar, and PsychInfo.
Results: 47 studies were included, 9 concerning cardiovascular disease, 18 neurodegenerative disorders, and 20 for cancer disease. According to the reviewed studies, people experience no significant increase in distress and anxiety, or adverse impacts on quality of life, except the Huntington disease, which is characterized by depressive symptoms, suicidal ideations, and hopelessness in gene carriers. People tend to consider genetic tests as valid information to take important preventive decisions. Genetic risk for cardiovascular disease is perceived to be manageable; genetic analysis for some neurodegenerative diseases (e.g., Alzheimer) or cancer (breast cancer in particular) is considered useful because the problem could be addressed in advance with preventive behaviors.
Conclusions: Genetic tests should be proposed along with proper psychological support and counseling focused on users' genetic health literacy; perception of risk, beliefs about disease controllability, in order to foster fruitful medical decisions.
Introduction
Cancer, cardiovascular diseases and dementia are among the main causes of mortality and morbidity in Europe. Since they will have even larger economic implications in the future, policy-makers have increasingly focused their attention on them (Abegunde et al., 2007; Désesquelles et al., 2014; Mackenbach et al., 2014; Suzman et al., 2015). These conditions affect many people worldwide often causing an impairment of the quality of life and psychosocial well-being, thus they require the attention of the scientific community. In 2015 cardiovascular disease death were 17.92 million, with 422.7 million of cases worldwide (Roth et al., 2017). The WHO estimated that 17.7 million people died from cardiovascular disease last year. Alzheimer disease (AD) affects approximately 24 million people globally (Erkkinen et al., 2018) and this number could quadruple by 2050. Currently, dementia is reported to be the leading cause of mortality in England and Wales (Office for National Statistics ONS), and in 2015 the Eurostat (the Directorate-General of the European Commission) reported 213,000 deaths in Europe caused by nervous system diseases including Alzheimer's.
Regarding cancer data, 14.1 million new cancer cases and 8.2 million cancer deaths occurred in 2012 worldwide (Ferlay et al., 2015), which in 2015 grew to 8.8 million (WHO).
These complex conditions usually need long-term treatments and care, involving different health professionals, expensive drugs, access to medical equipment, putting a large burden on society.
The growing diagnoses of many chronic diseases are associated with an aging population, but also with lifestyle choices such as smoking, diet and exercise, and genetic predisposition (WHO and FAO, 2003; World Health Organisation, 2014).
In the last decades, there have been considerable investments in genomics (DNA-based) research to study susceptibility to cancer and other chronic diseases and to promote new preventive interventions (Walter and Emery, 2012). Currently, the use of family health history and multiplex genetic tests to identify an individual's risk for multiple diseases simultaneously is a frequent clinical practice (Yang et al., 2003; Yoon and Scheuner, 2003; Khoury et al., 2004; Yoon, 2005). Many associations between single-nucleotide polymorphisms (SNPs) and risks for common complex diseases have been identified. Genetic testing generally provides information about the presence of these genetic variants (SNPs), which could represent an increased risk of developing the disease. Their clinical utility depends on how much the knowledge about this genetic variant could give additional information concerning diagnosis, prognosis or contribute to disease management. Available types of testing include for instance diagnostic, carrier, predictive and susceptibility tests. Diagnostic tests confirm a diagnosis when a particular condition is suspected, based on physical symptoms. Carrier testing identifies people who carry one copy of a gene mutation that can be inherited by their offspring. Predictive and susceptibility testing identifies mutations that increase a person's risk of developing disorders with a genetic basis. These tests may help people making decisions about their daily habits or medical care. For instance, discovering the susceptibility for breast cancer, or stroke could allow people to change their lifestyle, nutrition and “take steps to reduce those risks for which interventions are or will be available” (Collins and McKusick, 2001). Nonetheless, not all kinds of genetic testing are useful for clinical management or outcomes improvement, either because of a lack of treatments, uselessness for the personal decision, or absence of scientific evidence for the genetic predisposition. Proven clinical utility and cost-effectiveness need to be carefully evaluated when considering the implementation of genetic testing in healthcare (Cornel et al., 2014), even when considering the recent discoveries which have underlined the heterogeneity of chronic diseases and the importance of gene-environment interaction in modulating disease onset and responses to preventive interventions (Curtis et al., 2012).
In spite of these premises, a recent study, published by the Market Research Future (the “Global Genetic Testing Market - Forecasts from 2018 to 2023” see https://www.marketresearchfuture.com/), reported the amount of genetic tests performed in the European and non-European countries based on the subdivision of pathologies or on the type of genetic test (diagnostic, predictive, etc.). The study estimated a global growth of genetic testing market at a Compounded Average Growth Rate (CAGR) of 12.94% by 2023. This exponential growth should go hand in hand with an appropriate genetic counseling practice, but to date genetic information is often given to people with poor genetic literacy without a specific psychological assessment (Burke et al., 2002) or genetic testing users do not receive a specific pre and post-test counseling (Janssens et al., 2017). For instance, in Italy, only 12% of all genetic analyses had been accompanied by pre or post-test counseling (Giardino et al., 2016).
The genetic counseling is usually provided by trained professionals, mainly geneticists, who explain the genetic aspects of illnesses and the risk of developing or passing an illness to their offspring (WHO). It should address patients' concerns, and one of their families, to help with the decision-making process. Nevertheless, this not always happened in recent years especially with the introduction of direct to consumers genetic testing (DTC), genetic tests sold to people without a medical intermediate (Oliveri and Pravettoni, 2016).
Starting from these premises, we could infer that genetic risk communication might deeply affect people's lives and habits. To date, studies on the psychological impact of genetic tests mainly focused on “harmful” reactions, such as anxiety, distress, and depression, when receiving genetic risk information, obtaining discordant results and without any explanation/discussion for this discrepancy (Oliveri et al., 2016a). Previous reviews revealed that DNA based disease risk has little or no effect on health-related behaviors (Heshka et al., 2008; Hollands et al., 2016). We should consider that genetic testing impact also depends on how people perceive their risk, severity, and controllability related to specific categories of disease (Cameron and Muller, 2009; Wang et al., 2009; Wade et al., 2012); on the genetic tests predictability or nature of the diseases (from monogenic to genetic susceptibility factors), and on the presence/absence of treatments (Cameron and Muller, 2009).
People's emotional reactions to genetic testing and how risk perceptions vary from diseases to disease are fundamental aspects to be investigated on, in order to correlate preventive behaviors they may, or may not, adopt (DiLorenzo et al., 2006; Shiloh et al., 2013).
For this reason, we aim to provide a comprehensive overview of studies realized in the last two decades (2000–2016), which investigated psychological and behavioral issues after having undergone genetic testing for different categories of chronic diseases. The purpose is to identify a limited number of overarching psychological reactions for each condition. In particular, we chose to compare neurodegenerative, cardiovascular and cancer diseases, because of their differences in treatments availability and preventive options (e.g., there are fewer preventive options for neurodegenerative disorders compared to cancer or cardiovascular diseases, where risk is in some cases manageable with screenings or healthier lifestyles). Moreover, people have different beliefs and perception of the controllability for these diseases which could affect their reaction to a positive genetic test result.
Methods
Study Design and Search Strategy
Potential eligible articles were systematically searched on PubMed, Google Scholar and PsychInfo using the following combinations of terms: “psychological outcomes,” “psychological impact,” “genetic test,” “genetic risk,” “neurodegenerative disorders,” “cancer,” and “cardiovascular disease.” Depending on the disease for which the genetic test was performed, we allocated the collected articles into three general categories: Cancer (C), Cardiovascular diseases (CV), and Neurodegenerative disorders (N) (see Table 1). Following criteria were considered to include articles:
(a) studies in which a psycho-behavioral and/or quality of life evaluation after having received genetic test results was performed;
(b) studies in which subjects tested were adults.
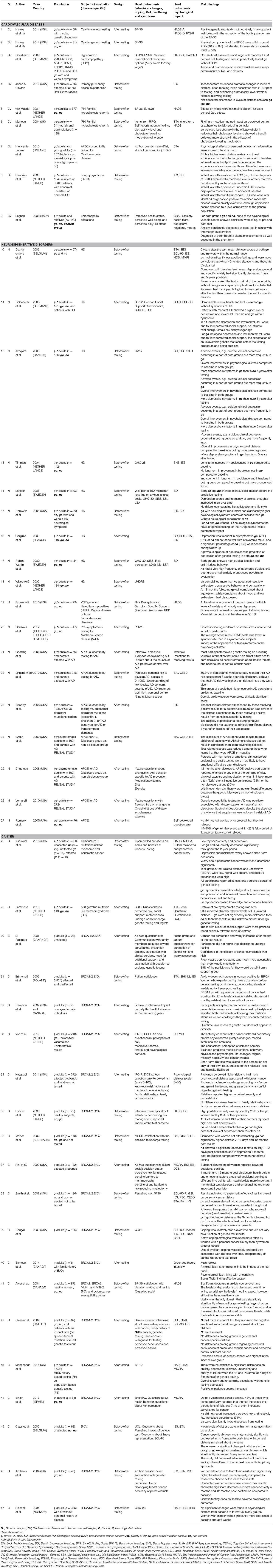
Table 1. Characteristics of studies evaluating psychological impact of genetic testing for cardiovascular, neurodegenerative and cancer diseases.
Exclusion criteria were determined by the aim to analyze the “raw” impact genetic test information can have on people's psychological reactions and/or quality of life, without the mediation of interventions (e.g., counseling), or specific population (e.g., children) or phenomenon (e.g., Direct to Consumer genetic testing).
Exclusion criteria were as follow:
(a) investigation of prenatal screening, or childhood and adolescent genetic testing;
(b) investigation of genetic testing for psychiatric disorders;
(c) investigation of family dynamics, the efficacy of psychological or other kind of educational and counseling intervention;
(d) hypothetical situations in undergoing genetic testing;
(e) direct to consumer genetic testing.
Recent reviews, meta-analyses or narrative accounts of knowledge were excluded.
For each study, we identified the implemented design, the number, and composition of participants, the psychological instruments employed and the main findings regarding psycho-behavioral outcomes and quality of life after testing.
Because of substantial heterogeneity among research studies, no attempt at formal meta-analysis was made in this contribution.
Results
Studies Selection and Characteristics
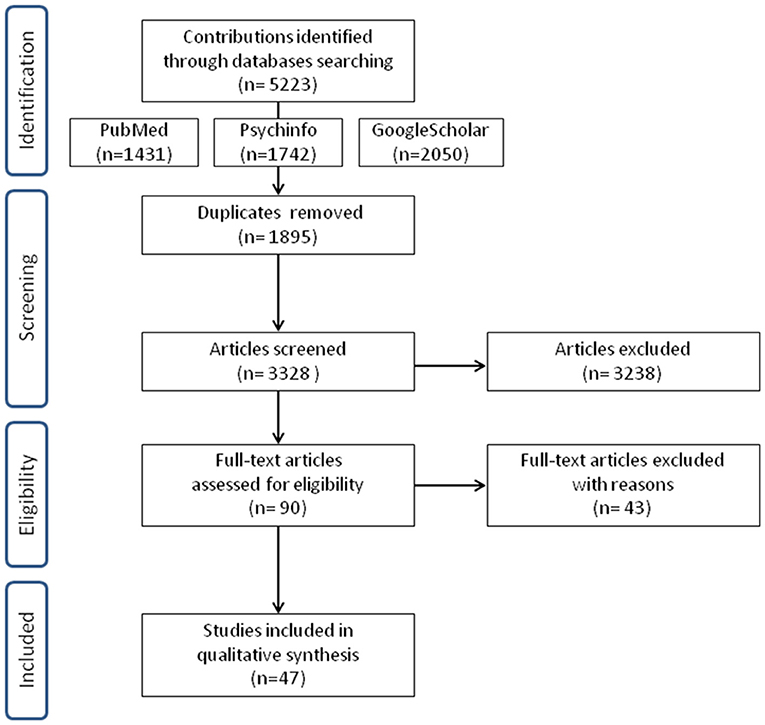
Three thousand and three hundred twenty-eight manuscripts, published between 2000 and 2016, were assessed for eligibility by reading title and abstract. In total 90 studies were potentially eligible. After reading the full text, articles were included for qualitative synthesis only if they met inclusion/exclusion criteria. As a consequence, 43 studies could not be included, mostly because they investigated prenatal screening or childhood diseases, psychiatric disorders, family dynamics, the efficacy of the psychological intervention, genetic counseling effects or hypothetical situations in undergoing genetic testing. We considered these studies suitable to be treated as a separate topic.
Finally, a set of 47 studies met our inclusion criteria and were completely assessed (see Figure 1 for study selection and Table 1 for a summary of selected studies). Nine studies are concerned with cardiovascular diseases, 18 neurodegenerative disorders, and 20 cancer diseases. They had been conducted in the United States and Canada (n = 22), Europe (n = 22), Australia (n = 1), Israel (n = 1) and the islands of Flores and S.Miguel (n = 1). Included studies have been classified according to the disease for which patients were tested (Table 1 first column), while the study design is reported in Table 2.
Cardiovascular Disease
Genetic testing for cardiovascular diseases is usually performed to detect users' susceptibility to conditions that affect the heart muscle, inherited heart conditions that might cause arrhythmias or risk factors which could cause a heart attack. Some of these conditions may require changes in lifestyle or medical therapy. Studies taken into examination evaluated the impact of genetic testing related to different clinical conditions (Hickey et al., 2014a,b), such as Long qt syndrome (Hendriks et al., 2008), thrombophilia (Legnani et al., 2006), cardiomyopathy (Christiaans et al., 2009), arterial hypertension (Jones and Clayton, 2012), and familial hypercholesterolaemia (Van Maarle et al., 2001; Marteau et al., 2004). In general, these studies used similar scales for the assessment of the quality of life and perception of risk (SF-36, IPQ-R) and for evaluating the psychological impact and wellbeing (STAI, HADS, IES). Psychological aspects mainly concerned the anxiety-depressive symptoms and the subjective distress caused by the “traumatic event” of genetic risk communication.
Results showed that no negative impacts on quality of life and no serious increase in distress or anxiety levels were registered after receiving genetic test results (Van Maarle et al., 2001; Marteau et al., 2004; Legnani et al., 2006; Hickey et al., 2014a,b). Anxiety levels were overall moderate and tended to last over time only if the genetic test result was associated with uncertain physiological data (Hendriks et al., 2008) or in case of marked clinical conditions, such as in patients with symptoms of hypertrophic cardiomyopathy before DNA testing (Christiaans et al., 2009). Hietaranta-Luoma et al. (2015) evaluated the impact of genetic testing for the Apolipoprotein E (ApoE, a protein involved in Alzheimer's disease and cardiovascular disease and mapped to chromosome 19). They reported that in high-risk subjects the genetic information combined with personal health status influenced the levels of anxiety, and promoted the short-term reduction of risk factors for cardiovascular disease. One study investigated arterial hypertension (Jones and Clayton, 2012) and detected distress symptoms in patients before undergoing genetic testing, comparable to PTSD symptoms. These symptoms significantly decreased after the reception of genetic results, in both carriers and non-carriers.
Even the quality of life after genetic test results was influenced by patients' difficulties in managing mental states, compared to other aspects. Hickey et al. (2014b) found that mental difficulties, assessed by the SF-36, were higher (59.9 ± 5.3) if compared to physical components, which resulted within normal ranges (46.2 ± 6.6), whereas Christiaans et al. (2009) clarified that levels of distress and impact on mental components did not significantly differ from the average of the general population who never underwent a genetic test. Finally, Marteau et al. (2004) showed that genetic testing for patients, already aware of their risk, does not affect their sense of control over the condition (hypercholesterolemia) but influenced their beliefs on how effectively achieve control on their health (e.g., with drug assumption).
Neurodegenerative Disorders
Genetic testing for neurodegenerative disorders are usually performed: (a) for diagnostic purposes, (b) to determine if a person, who has a family history of disease, is a mutation carrier and thus he/she is at risk to develop the disorder or could have an affected offspring. Currently, no therapies exist for complete remission of these pathologies. Studies we have collected primarily investigated risk related to Alzheimer disease (AD) and Huntington disease (HD). Two studies devoted attention to other neurodegenerative disorders, such as the Machado-Joseph disease (MJD)(Gonzalez et al., 2012) (which causes symptoms like spasticity, difficulty with speech and swallowing, weakness in arms and legs, frequent urination) and mutation to VCP gene (Surampalli et al., 2015) (which along with the inclusion body myopathy it causes frontotemporal dementia).
Huntington Disease (HD) is a dominantly transmitted neurodegenerative disorder: genetic analysis detects, with 100% of certainty, the presence of the mutation gene, confirming the status as a carrier of the condition (Evers-Kiebooms and Decruyenaere, 1998). The outcomes of gene testing can rarely fall within the reduced penetrance range (36–39 CAG repeats), whereby individuals may or may not develop symptoms of the disease; or individuals may be carriers of intermediate alleles (27–35 CAG repeats) and will not develop symptoms of the disease themselves, but their children will be at-risk of HD (Myers, 2004).
Tools used to evaluate the psychological impact of genetic testing for HD predominantly measured anxiety and depression (STAI, BDI), the traumatic impact of the event “genetic test results communication” (IES) or severe psychological symptoms, up to suicidal ideations (see Table 1).
Most authors highlighted the presence of depression and suicidal ideation in a significant percentage of participants, even before undergoing genetic testing (Robins Wahlin et al., 2000; Horowitz et al., 2001; Larsson et al., 2006; Gargiulo et al., 2009), with higher psychological suffering and negative impact on QoL for those with neurological symptoms (Horowitz et al., 2001). Licklederer et al. (2008) found that patients, with mutation and already manifesting HD symptoms, had higher levels of depression and lower levels in QoL indexes, compared to gene carriers without symptoms and non-carriers. Moreover, they showed that depression values in HD gene carriers were related to unfavorable genetic test result in conjunction with negative social and relational conditions (e.g., low perceived social support and being childless). Summarizing, higher levels of depression and lower quality of life were registered in patients with manifest HD or neurological impairments (Horowitz et al., 2001; Licklederer et al., 2008).
Interestingly, Gargiulo et al. (2009) found that 27% of non-carriers (asymptomatic) do not positively elaborate the favorable genetic results whereas Robins Wahlin et al. (2000) showed that non-carriers had a very high frequency of suicide ideations. Another study has also shown that non-carriers tended to develop avoidant or intrusive styles as a reaction to the stressful event (genetic test results) over time (Timman et al., 2004).
Considering the long-term impact of genetic tests for HD, several studies revealed the presence or the increase in depressive symptoms, suicidal ideations, hopelessness, and aggressive reactions in gene carriers (Robins Wahlin et al., 2000; Witjes-Ané et al., 2002; Almqvist et al., 2003; Timman et al., 2004; Larsson et al., 2006; Gargiulo et al., 2009), except for the study of Decruyenaere et al. (2003), showing a significant decrease of depressive symptoms after 1 year, both in gene carriers and non-carriers.
Concerning Alzheimer disease (AD), currently ApoE testing is used in clinical settings to identify people who may have an increased risk of developing AD, whereas other genetic tests investigate the presence of autosomal dominant mutations (in genes PSEN1, PSEN2, and APP which are more predictive for disease development (Goldman et al., 2011).
The REVEAL studies (Chao et al., 2008; Green et al., 2009; Vernarelli et al., 2010) showed that ApoE carriers were not more anxious, depressed, or test-related distressed than people who did not receive any information about their genotype (Green et al., 2009). The levels of anxiety, depression, and distress were below clinical thresholds both in carriers and non-carriers, with a significant distress reduction among those who learned that they were ApoE negative. People who were highly distressed before undergoing genetic testing were more vulnerable to emotional difficulties after outcome disclosure, but distress values were well below clinical thresholds for clinical concern (Green et al., 2009). Romero et al. (2005) described that a small percentage of ApoE gene carriers felt depressed (15–30%) or worried (11–22%). A small percentage also felt relieved.
The study by Cassidy et al. (2008) found that participants who received a positive result for a deterministic mutation experienced the same levels of distress experienced by those receiving positive results for genetic susceptibility testing (ApoE). The same study reported that after 1 year from result disclosure the majority of participants did not experience clinically significant distress.
Concerning more in detail long-term results or changes in health-related behaviors, 12 months after ApoE results, carriers reported changes in lifestyle (diet, physical exercise, and medication or vitamin intake) more often than non-carriers or the nondisclosure group (Chao et al., 2008). A positive correlation between genetic susceptibility testing for AD (an APOE epsilon4+ genotype status) and changes in vitamin intake was also confirmed by Vernarelli et al. (2010), despite there is no evidence that supplement use reduces the risk of AD.
Finally, Linnenbringer et al. (2010) showed that people who accurately recalled their AD disease risk assessment (the risk percentage) tended to perceive their risk higher than the percentage of risk they were given (below clinical thresholds).
Finally, Gooding et al. (2006) interviewed a group of people at high risk for AD (because of relatives affected by AD), and genetic testing was estimated valuable information to improve personal control on health and guide future decisions.
In rare pathologies such as Machado Joseph disease (Gonzalez et al., 2012), anxiety levels were from moderate to severe in half of the participants (52.6%). Five years later quality of life was significantly more compromised in symptomatic people, confirming an impact of the appearance of first symptoms on the psychological state. Meanwhile in VCP genetic testing (Surampalli et al., 2015) were found similar results in anxiety levels as for Alzheimer disease.
Cancer
Genetic testing for cancer is usually performed in pre-symptomatic conditions (the user never developed any symptom related to the cancer disease), or after an episode of cancer diagnosis, to know if there is a hereditary cancer syndrome and/or a risk of relapse.
Most of the articles focused on the risk of developing ovarian and breast cancer, by examining the presence of BRCA1 and BRCA2 mutations (see Table 1). Three studies, respectively, investigated the impact of genetic mutations responsible for pancreatic cancer and melanoma (Aspinwall et al., 2013), colon cancer along with breast cancer (Arver et al., 2004), and Li-Fraumeni Syndrome (Lammens et al., 2010). Studies on BRCA testing used several tools (STAI, IES, SCL-90, BDI, HADS the most used ones) assessing anxiety, post-traumatic stress disorders, psychopathological symptoms and depression, and showed quite heterogeneous results.
Breast and ovarian cancer were overall perceived as having the same seriousness independently by genetic test results (Claes et al., 2004). Many authors revealed the poor influence of genetic tests on anxiety and distress (distress levels within normal ranges), without significant difference between gene carriers and non-carriers (Andrews et al., 2004; Claes et al., 2005; Ertmanski et al., 2009). These results suggest that genetic testing for BRCA does not cause adverse psychological reactions. Four studies reported slightly greater levels of anxiety and negative psychological outcomes in gene carriers (Lodder et al., 2001a; Meiser et al., 2002; Katapodi et al., 2011; Shiloh et al., 2013) whereas Vos et al. (2012) specified that these anxiety levels would be mediated by individual risk perception and concerns about their own relatives' heredity-likelihood. Gene carriers and probands showed to be more distressed and negatively influenced by genetic test results, even because they were concerned about their offspring and experienced decisional conflicts toward their relatives (Claes et al., 2004; Rini et al., 2009; Katapodi et al., 2011). Three studies (Reichelt et al., 2004; Ertmanski et al., 2009; Manchanda et al., 2015) investigated the experience of genetic testing and risk perception in people with a family history or personal history of illness, comparing them with healthy people or people without previous family experience of disease, and they found conflicting results. Manchanda et al. (2015) demonstrated that there were no differences in levels of anxiety and distress based on the presence/absence of a family history of disease, while Reichelt et al. (2004) and Ertmanski et al. (2009) reported higher levels of distress in people who have already had a diagnosis and/or cancer experience. Women with a personal cancer history tended to enact concrete coping strategies more than women without previous experience with cancer (Dougall et al., 2009).
Finally, there are studies which reported satisfaction and positive consequences of having carried out the genetic test for breast/ovarian cancer susceptibility and thus discovering something about the presence of a mutation. In particular, receiving a positive result increased the perception of risk (Di Prospero et al., 2001; Claes et al., 2005; Katapodi et al., 2011; Vos et al., 2012), which correlated with more frequent screenings and checkups, and with a sense of self-efficacy (Di Prospero et al., 2001; Hamilton et al., 2009; Shiloh et al., 2013).
Long-term results showed that levels of test-related distress decreased in the first 4/6 months (Andrews et al., 2004; Arver et al., 2004; Smith et al., 2008), then enduring at low levels after years (Andrews et al., 2004; Manchanda et al., 2015), with an impact on surveillance actions up to 4 years after test disclosure (Shiloh et al., 2013).
Concerning other cancers, in Aspinwall et al. (2013) gene carriers for pancreatic cancer and melanoma increased preventive screening for themselves and their families, thanks to informative genetic test results. A small percentage of patients reported clinically relevant levels of distress related to genetic testing for p53 germline mutation (Lammens et al., 2010). Distress was higher for patients with a lack of social support, as was the case for Huntington Disease (Licklederer et al., 2008).
Discussion
In the last decades, clinical application of genetic testing for diagnosis and prevention has gained more importance to such an extent as to create a market where patients can obtain information on genetic risk in complete autonomy (Su, 2013; Oliveri et al., 2015, 2016b; Oliveri and Pravettoni, 2016). In this framework, there are many possible psychological reactions and related issues worthy of consideration, such as risk perception and perceived controllability after a positive result for a mutation, or concerns about transmitting susceptibility for a disease to future generations.
With this contribution, we aimed to sound out possible differences in psychological reactions to predictive genetic testing based on different disease categories. To date, there are no reports that compare the psychological impact of genetic testing for cardiovascular, neurodegenerative and cancer diseases.
Our review shows that there is no significant increase in distress levels or adverse impact on the quality of life in subjects who undergo a genetic test for cardiovascular diseases; when higher distress is present it does not exceed the clinically significant threshold (Van Maarle et al., 2001; Marteau et al., 2004; Legnani et al., 2006; Hickey et al., 2014b). The psychological distress is related to a full-blown clinical condition in addition to a positive genetic result (Hendriks et al., 2008; Christiaans et al., 2009). Overall people maintain confidence in being able to cope with their risk, even though they modify the opinion on how to address this risk: they tend to believe that lifestyle might be useless to face their “genetic predisposition,” and they need other “more concrete” methods of prevention, such as drug therapies (Marteau et al., 2004). In our opinion, these trends arise from a “deterministic” interpretation of genetic data, and the lack of evidence concerning the effects of lifestyle modifications in the disease course. Changes in lifestyle only concern people who already have physical symptoms (Marteau et al., 2004; Hietaranta-Luoma et al., 2015) and are at higher risk of adverse heart conditions, although these lifestyle changes have short duration (Hietaranta-Luoma et al., 2015). We hypothesize that people with full-blown symptoms are motivated to gather all possible health-related information, including genetic risk information, in order to manage their risk of developing the disease. In general, our review shows that genetic risk for cardiovascular disease is perceived to be manageable, and this might also be due to the existence of screenings to prevent it and possible treatments.
Concerning neurodegenerative disorders, studies put more attention on anxiety and depression symptoms, since these disorders usually have relevance on complex emotions such as embarrassment and social withdrawal (Levenson et al., 2014), affect family relations and put carriers at risk of social discrimination (Perry, 1981; Craufurd and Harris, 1986). Our review describes marked negative psychological impact after positive genetic results for Huntington Disease in patients who have depressive symptoms already before undergoing genetic testing, including suicidal ideation, which are increased also by the presence of adverse relational/family situations (Robins Wahlin et al., 2000; Horowitz et al., 2001; Larsson et al., 2006; Licklederer et al., 2008; Gargiulo et al., 2009). Differently from other chronic diseases, a negative genetic result for HD does not reassure, but it causes negative emotions. This reaction might be due to the uncertainty of results and a lack of “response” for the etiology of cognitive symptoms, when present, or sense of guilty toward family members that have the diseases (Robins Wahlin et al., 2000; Timman et al., 2004; Gargiulo et al., 2009). However, we believe that these results for Huntington disease should be taken with the due caution called by the fact that, often, the evidence was based on participants with previous psychiatric history (Robins Wahlin et al., 2000; Almqvist et al., 2003; Larsson et al., 2006; Gargiulo et al., 2009). Thus, we cannot rule out that, for example, a manifestation of suicidal ideation can be ascribable to this previous psychiatric history rather than to the positive or negative genetic result. Future studies should settle this issue.
Negative psychological impact of genetic testing in gene carriers for HD persists over time (Almqvist et al., 2003; Timman et al., 2004; Larsson et al., 2006), and it might be due to the regret for having undergone the test, anticipating life change limitations (Hagberg et al., 2011).
We argue that in addition to the regret for getting such genetic information, negative reactions may be understandable in light of the certainty these people have to develop HD in the future, the perception of something uncontrollable and fatal, alongside the complete absence of valid therapies and inevitable cognitive decline (Gooding et al., 2006). The decision in undergoing predictive genetic testing, in this case, could be a coping strategy (Gooding et al., 2006)acted to redirect important life decisions.
Slightly different seems to be the impact of genetic analysis for Alzheimer disease. Effects of genetic test results are comparable to those described for cardiovascular diseases, since distress anxiety and depression are below clinically significant thresholds, even for gene carriers, and these results concern both APOE and autosomal dominant mutation testing (Cassidy et al., 2008; Green et al., 2009; Linnenbringer et al., 2010). Therefore, the test is overall experienced as something useful to achieve a good degree of awareness and immediately act preventive behaviors to address the risk. Anyway, for Alzheimer's prevention behavioral changes are not always positive: for example, an increasing assumption of dietary supplements harmless, such as vitamin E, could give people a false perception of control on the health without any significant scientific evidence (Morris et al., 2002). For this reason, it would be beneficial to provide people with more information on how to effectively prevent AD, before providing the opportunity to undergo genetic testing.
Finally, in rare diseases, such as Machado Joseph, anxiety levels were prominent after genetic testing in at least half of patients studied (Gonzalez et al., 2012). These results are understandable in the light of an immediate impairment of daily life (e.g., spasticity, difficulty with speech and swallowing, weakness in arms and legs, frequent urination) and the fact that symptoms get worse over time.
Results on the impact of gene testing in cancer raise more complex and heterogeneous issues than in previous cases. From the emotional point of view, the levels of anxiety and depression decrease significantly after having received test results (Andrews et al., 2004; Arver et al., 2004; Reichelt et al., 2004; Claes et al., 2005; Smith et al., 2008; Ertmanski et al., 2009; Lammens et al., 2010; Aspinwall et al., 2013; Manchanda et al., 2015), and a positive effect emerges as regards screening behaviors as well (Hamilton et al., 2009; Shiloh et al., 2013). We must consider, in order to give an interpretation to the previous results, that breast and ovarian cancer are potentially preventable and early detection can guarantee to heal with effective treatments (Shaw and Bassi, 2001). If results are positive, screening or surgery could help patients reduce their risks, and immediate communication to family members about genetic risk can be crucial to prevent the “danger” of disease development. Deciding on how to address the risk means being able to “recommend” a pathway for prevention to their families (Lodder et al., 2001b; Katapodi et al., 2011; Vos et al., 2012). Preventive and prophylactic decisional pathways are no easy nor straight: risk-reducing prophylactic mastectomy on healthy breast goes along the risk of surgical side effects, body image modification, regrets in women who decided for this solution. Periodic screening is instead potentially accompanied by frequent negative thoughts and emotions (anxiety components).
People who have already had an experience of illness tend to actively cope with the risk of disease onset, although sometimes this is accompanied by higher levels of distress (Dougall et al., 2009). These levels of arousal, however, should not be necessarily perceived in a negative sense; on the contrary emotional arousal could be the engine for the decisional process in cancer care and for acting on coping strategies. Even for cancer, as already found for APOE and Alzheimer's, studies indicate that there are positive aspects reported by patients about having undergone genetic testing. These findings are related to an increase in screening behaviors and an increased sense of self-efficacy in managing the risk (Hamilton et al., 2009; Aspinwall et al., 2013; Shiloh et al., 2013). Summarizing, in cancer, if people get important information on time they can manage their risky or healthy behaviors enhancing the perception of control over their lives and direct it as they wish (e.g., surgery vs. screening).
This is not completely true for cardiovascular disease and Huntington disease, because cardiovascular disease genetic information and evidence about preventive medicine efficacy are completely uncertain and say “something less” about predisposition and prevention options; for HD, deterministic implications of genetic testing give a piece of information that is likely to be “too much information,” and thus perceived as uncontrollable.
Before to conclude, it is important to point out some limitations of the reviewed studies. The first limitation is that gender was not well balanced in all the selected studies and was not investigated as a factor that could influence the psycho-behavioral impact of genetic testing. We exclude papers on breast cancer (which investigated women samples), Li-Fraumeni Syndrome (LFS) (where men and female were equally balanced and female gender was associated with heightened levels of LFS-related distress), and on the risk for developing diseases which disproportionately affect women (e.g., the primary pulmonary arterial hypertension)(Jones and Clayton, 2012). In many studies about neurodegenerative diseases, women were overrepresented (Cassidy et al., 2008; Green et al., 2009; Vernarelli et al., 2010; Gonzalez et al., 2012) and we cannot exclude that the results could be affected by general gender bias. Chao et al. (2008), for example, claimed that the REVEAL study participants were mainly women. Therefore the results may not be generalized to all population who might qualify for APOE genotype testing in the future.
In most studies concerning cardiovascular disease, males and female samples seemed to be well balanced, except for Hietaranta-Luoma et al. (2015) who evaluated more females. Nevertheless, gender differences, when investigated (Legnani et al., 2006; Jones and Clayton, 2012; Hickey et al., 2014a) did not relate to any of the measures of patient well-being.
A second limit was that not all the studies considered participants' educational level as a factor that could correlate with the decision to undergo a genetic test and its psycho-behavioral impact. In the selected studies participants had at least high school education (10–12 years of education completed)(Lodder et al., 2001b; Van Maarle et al., 2001; Witjes-Ané et al., 2002; Almqvist et al., 2003; Claes et al., 2004; Gooding et al., 2006; Legnani et al., 2006; Licklederer et al., 2008; Smith et al., 2008; Christiaans et al., 2009; Rini et al., 2009; Vernarelli et al., 2010; Gonzalez et al., 2012; Vos et al., 2012; Hickey et al., 2014b) or were predominantly highly educated (Andrews et al., 2004; Claes et al., 2005; Dougall et al., 2009; Vernarelli et al., 2010; Aspinwall et al., 2013; Shiloh et al., 2013; Manchanda et al., 2015). Educational level was associated to a better recall of disease risk information (Linnenbringer et al., 2010), to a higher response rates in the follow up (Almqvist et al., 2003), or was not an influential predictor of the psycho-behavioral measures (Robins Wahlin et al., 2000; Meiser et al., 2002; Andrews et al., 2004; Claes et al., 2005; Legnani et al., 2006; Licklederer et al., 2008; Smith et al., 2008; Green et al., 2009; Rini et al., 2009; Jones and Clayton, 2012; Shiloh et al., 2013). Other studies included in our review did not perform analysis based on the educational level, and future studies should address this issue.
Finally, some of the results presented in these studies are based on small sample sizes (Robins Wahlin et al., 2000; Di Prospero et al., 2001; Andrews et al., 2004; Hamilton et al., 2009; Gonzalez et al., 2012; Hickey et al., 2014b; Samson et al., 2014; Surampalli et al., 2015) and are not cross-cultural. Therefore, it is possible that ethnicity and cultural aspects may play a role in determining the psychological implications of genetic testing.
Conclusions
This review presented a comprehensive overview of the psychological impact of genetic testing across the most common chronic adults' diseases. The information level of genetic data varies according to the type of test. Along with this aspect, each of us has a specific perception of disease categories, for which genetic testing is available. Risk perception, worry, and other psychological reactions depend, for instance, on the perceived controllability and existing therapies to manage the illness; it is essential to proceed with an assessment of such factors along with the provision of genetic information.
Over the last 20 years we have witnessed a proliferation of investments in genomics research in order to study disease prevention, disease treatments, better drug therapies, and genetic paths to cure, and, thanks to media coverage such as Angelina Jolie's case (Evans et al., 2014), the psychological impact of these discoveries has gradually become more and more important.
For these reasons, in the present review, we tried to understand better how genetic testing users' perceptions of developing specific diseases affect their psychological well-being and lifestyle. Understand psycho-behavioral reactions could be an important starting point for an effective clinical application of genetic testing and to organize personalized care plans, which can drive patients to self-determination of a healthy lifestyle and to make appropriate decisions for their health.
Author Contributions
SO and FF contributed to the design and implementation of the research, to the analysis of the results and the writing of the manuscript. AM contributed to the writing of the manuscript, and GP supervised all the process, was in charge of overall direction and planning.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by a grant to the project Mind the Risk from The Swedish Foundation for Humanities and Social Sciences which did not influence the content of this paper. Grant nr. M13-0260:1.
References
Abegunde, D. O., Mathers, C. D., Adam, T., Ortegon, M., and Strong, K. (2007). The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 370, 1929–1938. doi: 10.1016/S0140-6736(07)61696-1
Almqvist, E. W., Brinkman, R. R., Wiggins, S., and Hayden, M. R. (2003). Psychological consequences and predictors of adverse events in the first 5 years after predictive testing for Huntington's disease. Clin. Genet. 64, 300–309. doi: 10.1034/j.1399-0004.2003.00157.x
Andrews, L., Meiser, B., Apicella, C., and Tucker, K. (2004). Psychological impact of genetic testing for breast cancer susceptibility in women of Ashkenazi Jewish background: a prospective study. Genet. Test. 8, 240–247. doi: 10.1089/gte.2004.8.240
Arver, B., Haegermark, A., Platten, U., Lindblom, A., and Brandberg, Y. (2004). Evaluation of psychosocial effects of pre-symptomatic testing for breast/ovarian and colon cancer pre-disposing genes: a 12-month follow-up. Fam. Cancer 3, 109–116. doi: 10.1023/B:FAME.0000039863.89137.f9
Aspinwall, L. G., Taber, J. M., Leaf, S. L., Kohlmann, W., and Leachman, S. A. (2013). Genetic testing for hereditary melanoma and pancreatic cancer: a longitudinal study of psychological outcome. Psychooncology. 22, 276–289. doi: 10.1002/pon.2080
Burke, W., Atkins, D., Gwinn, M., Guttmacher, A., Haddow, J., Lau, J., et al. (2002). Genetic test evaluation: Information needs of clinicians, policy makers, and the public. Am. J. Epidemiol. 156, 311–318. doi: 10.1093/aje/kwf055
Cameron, L. D., and Muller, C. (2009). Psychosocial aspects of genetic testing. Curr. Opin. Psychiatry 22, 218–223. doi: 10.1097/YCO.0b013e3283252d80
Cassidy, M. R., Roberts, J. S., Bird, T. D., Steinbart, E. J., Cupples, L. A., Chen, C. A., et al. (2008). Comparing test-specific distress of susceptibility versus deterministic genetic testing for Alzheimer's disease. Alzheimer's Dement. 4, 406–413. doi: 10.1016/j.jalz.2008.04.007
Chao, S., Roberts, J. S., Marteau, T. M., Silliman, R., Cupples, L. A., and Green, R. C. (2008). Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Dis. Assoc. Disord. 22, 94–97. doi: 10.1097/WAD.0b013e31815a9dcc
Christiaans, I., Van Langen, I. M., Birnie, E., Bonsel, G. J., Wilde, A. A. M., and Smets, E. M. A. (2009). Quality of life and psychological distress in hypertrophic cardiomyopathy mutation carriers: a cross-sectional cohort study. Am. J. Med. Genet. Part A 149, 602–612. doi: 10.1002/ajmg.a.32710
Claes, E., Evers-Kiebooms, G., Boogaerts, A., Decruyenaere, M., Denayer, L., and Legius, E. (2004). Diagnostic genetic testing for hereditary breast and ovarian cancer in cancer patients: women's looking back on the pre-test period and a psychological evaluation. Genet. Test. 8, 13–21. doi: 10.1089/109065704323015996
Claes, E., Evers-Kiebooms, G., Denayer, L., Decruyenaere, M., Boogaerts, A., Philippe, K., et al. (2005). Predictive genetic testing for hereditary breast and ovarian cancer: psychological distress and illness representations 1 year following disclosure. J. Genet. Couns. 14, 349–363. doi: 10.1007/s10897-005-1371-4
Collins, F. S., and McKusick, V. A. (2001). Implications of the Human Genome Project for medical science. JAMA 285, 540–544. doi: 10.1001/jama.285.5.540
Cornel, M. C., Van El, C. G., and Borry, P. (2014). The challenge of implementing genetic tests with clinical utility while avoiding unsound applications. J. Community Genet. 5, 7–12. doi: 10.1007/s12687-012-0121-1
Craufurd, D. I., and Harris, R. (1986). Ethics of predictive testing for Huntington's chorea: the need for more information. Br. Med. J. (Clin. Res. Ed). 293, 249–251.
Curtis, C., Shah, S. P., Chin, S.-F., Turashvili, G., Rueda, O. M., Dunning, M. J., et al. (2012). The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352. doi: 10.1038/nature10983
Decruyenaere, M., Evers-Kiebooms, G., Cloostermans, T., Boogaerts, A., Demyttenaere, K., Dom, R., et al. (2003). Psychological distress in the 5-year period after predictive testing for Huntington's disease. Eur. J. Hum. Genet. 11, 30–38. doi: 10.1038/sj.ejhg.5200913
Désesquelles, A., Demuru, E., Pappagallo, M., Salvatore, M. A., Frova, L., Meslé, F., et al. (2014). Mortality from Alzheimer' s disease, Parkinson' s disease, and dementias in france and Italy : a comparison using the multiple approach. J. Aging Health 26, 283–315. doi: 10.1177/0898264313514443
Di Prospero, L. S., Seminsky, M., Honeyford, J., Doan, B., Franssen, E., Meschino, W., et al. (2001). Psychosocial issues following a positive result of genetic testing for BRCA1 and BRCA2 mutations: findings from a focus group and a needs-assessment survey. CMAJ 164, 1005–1009. doi: 10.1002/ijc.23340
DiLorenzo, T. A., Schnur, J., Montgomery, G. H., Erblich, J., Winkel, G., and Bovbjerg, D. H. (2006). A model of disease-specific worry in heritable disease: The influence of family history, perceived risk and worry about other illnesses. J. Behav. Med. 29, 37–49. doi: 10.1007/s10865-005-9039-y
Dougall, A. L., Smith, A. W., Somers, T. J., Posluszny, D. M., Rubinstein, W. S., and Baum, A. (2009). Coping with genetic testing for breast cancer susceptibility. Psychosom. Med. 71, 98–105. doi: 10.1097/PSY.0b013e318190d7b4
Erkkinen, M. G., Kim, M.-O., and Geschwind, M. D. (2018). Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 10:a033118. doi: 10.1101/cshperspect.a033118
Ertmanski, S.ł., Metcalfe, K., TrempaŁa, J., GŁowacka, M. D., Lubinski, J., Narod, S. A., et al. (2009). Identification of patients at high risk of psychological distress after BRCA1 genetic testing. Genet. Test. Mol. Biomarkers 13, 325–330. doi: 10.1089/gtmb.2008.0126
Evans, D. G., Barwell, J., Eccles, D. M., Collins, A., Izatt, L., Jacobs, C., et al. (2014). The Angelina Jolie effect: how high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res. 16, 442. doi: 10.1186/s13058-014-0442-6
Evers-Kiebooms, G., and Decruyenaere, M. (1998). Predictive testing for Huntington's disease: a challenge for persons at risk and for professionals. Patient Educ. Couns. 35, 15–26.
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. doi: 10.1002/ijc.29210
Gargiulo, M., Lejeune, S., Tanguy, M.-L., Lahlou-Laforêt, K., Faudet, A., Cohen, D., et al. (2009). Long-term outcome of presymptomatic testing in Huntington disease. Eur. J. Hum. Genet. 17, 165–171. doi: 10.1038/ejhg.2008.146
Giardino, D., Mingarelli, R., Lauretti, T., Amoroso, A., Larizza, L., and Dallapiccola, B. (2016). Survey of medical genetic services in Italy: year 2011. BMC Health Serv. Res. 16:96. doi: 10.1186/s12913-016-1340-7
Goldman, J. S., Hahn, S. E., Catania, J. W., LaRusse-Eckert, S., Butson, M. B., Rumbaugh, M., et al. (2011). Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet. Med. 13, 597–605. doi: 10.1097/GIM.0b013e31821d69b8
Gonzalez, C., Gomes, E., Kazachkova, N., Bettencourt, C., Raposo, M., Kay, T. T., et al. (2012). Psychological well-being and family satisfaction levels five years after being confirmed as a carrier of the Machado-Joseph disease mutation. Genet. Test. Mol. Biomarkers 16, 1363–1368. doi: 10.1089/gtmb.2011.0370
Gooding, H. C., Linnenbringer, E. L., Burack, J., Roberts, J. S., Green, R. C., and Biesecker, B. B. (2006). Genetic susceptibility testing for Alzheimer disease: Motivation to obtain information and control as precursors to coping with increased risk. Patient Educ. Couns. 64, 259–267. doi: 10.1016/j.pec.2006.03.002
Green, R. C., Roberts, J. S., Cupples, L. A., Relkin, N. R., Whitehouse, P. J., Brown, T., et al. (2009). Disclosure of APOE genotype for risk of Alzheimer's disease. N. Engl. J. Med. 361, 245–254. doi: 10.1056/NEJMoa0809578
Hagberg, A., Bui, T. H., and Winnberg, E. (2011). More appreciation of life or regretting the test? Experiences of living as a mutation carrier of Huntington's disease. J. Genet. Couns. 20, 70–79. doi: 10.1007/s10897-010-9329-6
Hamilton, R., Williams, J. K., Skirton, H., and Bowers, B. J. (2009). Living with genetic test results for hereditary breast and ovarian cancer. J. Nurs. Scholarsh. 41, 276–283. doi: 10.1111/j.1547-5069.2009.01279.x
Hendriks, K. S. W. H., Hendriks, M. M. W. B., Birnie, E., Grosfeld, F. J. M., Wilde, A., van den Bout, J., et al. (2008). Familial disease with a risk of sudden death: a longitudinal study of the psychological consequences of predictive testing for long QT syndrome. Heart Rhythm 5, 719–724. doi: 10.1016/j.hrthm.2008.01.032
Heshka, J. T., Palleschi, C., Howley, H., Wilson, B., and Wells, P. S. (2008). A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet. Med. 10, 19–32. doi: 10.1097/GIM.0b013e31815f524f
Hickey, K. T., Sciacca, R. R., Biviano, A. B., Whang, W., Dizon, J. M., Garan, H., et al. (2014a). The effect of cardiac genetic testing on psychological well-being and illness perceptions. Hear. Lung J. Acute Crit. Care 43, 127–132. doi: 10.1016/j.hrtlng.2014.01.006
Hickey, K. T., Taylor, J. Y., Sciacca, R. R., Aboelela, S., Gonzalez, P., Castillo, C., et al. (2014b). Cardiac genetic testing: a single-center pilot study of a Dominican population. Hisp. Health Care Int. 12, 183–188. doi: 10.1891/1540-4153.12.4.183
Hietaranta-Luoma, H.-L., Luomala, H. T., Puolijoki, H., and Hopia, A. (2015). Using ApoE genotyping to promote healthy lifestyles in Finland–psychological impacts: randomized controlled trial. J. Genet. Couns. 24, 908–921. doi: 10.1007/s10897-015-9826-8
Hollands, G. J., French, D. P., Griffin, S. J., Prevost, A. T., Sutton, S., King, S., et al. (2016). The impact of communicating genetic risks of disease on riskreducing health behaviour: Systematic review with meta-analysis. BMJ 352. doi: 10.1136/bmj.i1102
Horowitz, M. J., Field, N. P., Zanko, A., Donnelly, E. F., Epstein, C., and Longo, F. (2001). Psychological impact of news of genetic risk for Huntington disease. Am. J. Med. Genet. 103, 188–192. doi: 10.1002/ajmg.1538
Janssens, S., Chokoshvili, D., Vears, D. F., De Paepe, A., and Borry, P. (2017). Pre- and post-testing counseling considerations for the provision of expanded carrier screening: Exploration of European geneticists' views. BMC Med. Ethics 18:46. doi: 10.1186/s12910-017-0206-9
Jones, D. L., and Clayton, E. W. (2012). The role of distress in uptake and response to predisposition genetic testing: the BMPR2 experience. Genet. Test. Mol. Biomarkers 16, 203–209. doi: 10.1089/gtmb.2011.0059
Katapodi, M. C., Northouse, L., Pierce, P., Milliron, K. J., Liu, G., and Merajver, S. D. (2011). Differences between women who pursued genetic testing for hereditary breast and ovarian cancer and their at-risk relatives who did not. Oncol. Nurs. Forum 38, 572–581. doi: 10.1188/11.ONF.572-581
Khoury, M. J., Yang, Q., Gwinn, M., Little, J., and Flanders, W. D. (2004). An epidemiologic assessment of genomic profiling for measuring susceptibility to common diseases and targeting interventions. Genet. Med. 6, 38–47. doi: 10.1097/01.GIM.0000105751.71430.79
Lammens, C. R. M., Aaronson, N. K., Wagner, A., Sijmons, R. H., Ausems, M. G. E. M., Vriends, A. H. J. T., et al. (2010). Genetic testing in Li-Fraumeni syndrome: Uptake and psychosocial consequences. J. Clin. Oncol. 28, 3008–3014. doi: 10.1200/JCO.2009.27.2112
Larsson, M. U., Luszcz, M., a, Bui, T.-H., and Wahlin, T.-B. R. (2006). Depression and suicidal ideation after predictive testing for Huntington's disease: a two-year follow-up study. J. Genet. Couns. 15, 361–374. doi: 10.1007/s10897-006-9027-6
Legnani, C., Razzaboni, E., Gremigni, P., Bitti, P. E. R., Favaretto, E., and Palareti, G. (2006). Psychological impact of testing for thrombophilic alterations. Thromb. Haemost. 96, 348–355. doi: 10.1160/TH06-01-0015
Levenson, R. W., Sturm, V. E., and Haase, C. M. (2014). Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annu. Rev. Clin. Psychol. 10, 581–606. doi: 10.1146/annurev-clinpsy-032813-153653
Licklederer, C., Wolff, G., and Barth, J. (2008). Mental health and quality of life after genetic testing for huntington disease: a long-term effect study in Germany. Am. J. Med. Genet. Part A 146, 2078–2085. doi: 10.1002/ajmg.a.32423
Linnenbringer, E., Roberts, J. S., Hiraki, S., Cupples, L. A., and Green, R. C. (2010). “I know what you told me, but this is what I think:” perceived risk of Alzheimer disease among individuals who accurately recall their genetics-based risk estimate. Genet. Med. 12, 219–227. doi: 10.1097/GIM.0b013e3181cef9e1
Lodder, L., Frets, P. G., Trijsburg, R. W., Meijers-Heijboer, E. J., Klijn, J. G. M., Duivenvoorden, H. J., et al. (2001a). Psychological impact of receiving a BRCA1/BRCA2 test result. Am. J. Med. Genet. Part A 98, 15–24.
Lodder, L., Frets, P. G., Willem Trijsburg, R., Johanna Meijers-Heijboer, E., Klijn, J. G. M., Duivenvoorden, H. J., et al. (2001b). Psychological impact of receiving a BRCA1/BRCA2 test result. Am. J. Med. Genet. 98, 15–24. doi: 10.1002/1096-8628(20010101)98:1<15::AID-AJMG1014>3.0.CO;2-0
Mackenbach, J. P., Karanikolos, M., and Looman, C. W. N. (2014). The rise of mortality from mental and neurological diseases in Europe, 1979-2009: observational study. BMC Public Health 14:840. doi: 10.1186/1471-2458-14-840
Manchanda, R., Loggenberg, K., Sanderson, S., Burnell, M., Wardle, J., Gessler, S., et al. (2015). Population testing for cancer predisposing BRCA1/BRCA2 mutations in the ashkenazi-jewish community: a randomized controlled trial. J. Natl. Cancer Inst. 107. doi: 10.1093/jnci/dju379
Marteau, T., Senior, V., Humphries, S. E., Bobrow, M., Cranston, T., Crook, M., et al. (2004). Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: a randomized controlled trial. Am. J. Med. Genet. A 128A, 285–293. doi: 10.1002/ajmg.a.30102
Meiser, B., Butow, P., Friedlander, M., Barratt, A., Schnieden, V., Watson, M., et al. (2002). Psychological impact of genetic testing in women from high-risk breast cancer families. Eur. J. Cancer 38, 2025–2031. doi: 10.1016/S0959-8049(02)00264-2
Morris, M. C., Evans, D., a, Bienias, J. L., Tangney, C. C., Bennett, D. a, Aggarwal, N., et al. (2002). Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 287, 3230–3237. doi: 10.1001/jama.287.24.3230
Oliveri, S., Howard, H. C., Renzi, C., Hansson, M. G., and Pravettoni, G. (2016a). Anxiety delivered direct-to-consumer: are we asking the right questions about the impacts of DTC genetic testing? J. Med. Genet. 53, 798–799. doi: 10.1136/jmedgenet-2016-104184
Oliveri, S., and Pravettoni, G. (2016). The disclosure of direct-to-consumer genetic testing: sounding out the psychological perspective of consumers. Biol. Med. 8, 1–4. doi: 10.4172/0974-8369.1000316
Oliveri, S., Pravettoni, G., Fioretti, C., and Hansson, M. G. (2016b). Let the individuals directly concerned decide: a solution to tragic choices in genetic risk information. Public Health Genomics 19, 307–313. doi: 10.1159/000448913
Oliveri, S., Renzi, C., Masiero, M., and Pravettoni, G. (2015). Living at risk: factors that affect the experience of direct-to-consumer genetic testing. Mayo Clin. Proc. 90, 1323–1326. doi: 10.1016/j.mayocp.2015.06.014
Reichelt, J. G., Heimdal, K., Møller, P., and Dahl, A. A. (2004). BRCA1 testing with definitive results: a prospective study of psychological distress in a large clinic-based sample. Fam. Cancer 3, 21–28. doi: 10.1023/B:FAME.0000026820.32469.4a
Rini, C., O'Neill, S. C., Valdimarsdottir, H., Goldsmith, R. E., Jandorf, L., Brown, K., et al. (2009). Cognitive and emotional factors predicting decisional conflict among high-risk breast cancer survivors who receive uninformative BRCA1/2 results. Health Psychol. 28, 569–578. doi: 10.1037/a0015205
Robins Wahlin, T. B., Backman, L., Lundin, A., Haegermark, A., Winblad, B., and Anvret, M. (2000). High suicidal ideation in persons testing for Huntington's disease. Acta Neurol Scand 102, 150–161. doi: 10.1034/j.1600-0404.2000.102003150.x
Romero, L. J., Garry, P. J., Schuyler, M., Bennahum, D. A., Qualls, C., Ballinger, L., et al. (2005). Emotional responses to APO E genotype disclosure for Alzheimer disease. J. Genet. Couns. 14, 141–150. doi: 10.1007/s10897-005-4063-1
Roth, G. A., Johnson, C., Abajobir, A., Abd-Allah, F., Abera, S. F., Abyu, G., et al. (2017). Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25. doi: 10.1016/j.jacc.2017.04.052
Samson, A., DiMillo, J., Thériault, A., Lowry, S., Corsini, L., Verma, S., et al. (2014). Living with the BRCA1 and BRCA2 genetic mutation: Learning how to adapt to a virtual chronic illness. Psychol. Health Med. 19, 103–114. doi: 10.1080/13548506.2013.779729
Shaw, J. S., and Bassi, K. L. (2001). Lay attitudes toward genetic Testing for susceptibility to inherited diseases. J. Health Psychol. 6, 405–423. doi: 10.1177/135910530100600404
Shiloh, S., Dagan, E., Friedman, I., Blank, N., and Friedman, E. (2013). A follow-up study on men tested for BRCA1/BRCA2 mutations: impacts and coping processes. Psychooncology. 22, 417–425. doi: 10.1002/pon.2106
Smith, A. W., Dougall, A. L., Posluszny, D. M., Somers, T. J., Rubinstein, W. S., and Baum, A. (2008). Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psychooncology 17, 767–773. doi: 10.1002/pon.1291
Su, P. (2013). Direct-to-consumer genetic testing: a comprehensive view. Yale J. Biol. Med. 86, 359–365.
Surampalli, A., Khare, M., Kubrussi, G., Wencel, M., Tanaja, J., Donkervoort, S., et al. (2015). Psychological impact of predictive genetic testing in VCP inclusion body myopathy, paget disease of bone and frontotemporal dementia. J. Genet. Couns. 24, 842–850. doi: 10.1007/s10897-015-9819-7
Suzman, R., Beard, J. R., Boerma, T., and Chatterji, S. (2015). Health in an ageing world—what do we know? Lancet 385, 484–486. doi: 10.1016/S0140-6736(14)61597-X
Timman, R., Roos, R., Maat-Kievit, A., and Tibben, A. (2004). Adverse effects of predictive testing for Huntington disease underestimated: long-term effects 7-10 years after the test. Health Psychol. 23, 189–197. doi: 10.1037/0278-6133.23.2.189
Van Maarle, M. C., Stouthard, M. E. A., d. Mheen, P. J., Klazinga, N. S., and Bonsel, G. J. (2001). How disturbing is it to be approached for a genetic cascade screening programme for familial hypercholesterolaemia?: psychological impact and screenees' views. Community Genet. 4, 244–252. doi: 10.1159/000064200
Vernarelli, J. A., Roberts, J. S., Hiraki, S., Chen, C. A., Cupples, L. A., and Green, R. C. (2010). Effect of Alzheimer disease genetic risk disclosure on dietary supplement use. Am. J. Clin. Nutr. 91, 1402–1407. doi: 10.3945/ajcn.2009.28981
Vos, J., Oosterwijk, J. C., Gomez-Garcia, E., Menko, F. H., Collee, M. J., van Asperen, C. J., et al. (2012). Exploring the short-term impact of DNA-testing in breast cancer patients: The counselees' perception matters, but the actual BRCA1/2 result does not. Patient Educ. Couns. 86, 239–251. doi: 10.1016/j.pec.2011.04.017
Wade, C. H., Shiloh, S., Woolford, S. W., Roberts, J. S., Alford, S. H., Marteau, T. M., et al. (2012). Modelling decisions to undergo genetic testing for susceptibility to common health conditions: an ancillary study of the Multiplex Initiative. Psychol. Health 27, 430–444. doi: 10.1080/08870446.2011.586699
Walter, F. M., and Emery, J. D. (2012). Genetic advances in medicine: has the promise been fulfilled in general practice? Br. J. Gen. Pract. 62, 120–121. doi: 10.3399/bjgp12X629955
Wang, C., O'Neill, S. M., Rothrock, N., Gramling, R., Sen, A., Acheson, L. S., et al. (2009). Comparison of risk perceptions and beliefs across common chronic diseases. Prev. Med. (Baltim). 48, 197–202. doi: 10.1016/j.ypmed.2008.11.008
Witjes-Ané, M. N. W., Zwinderman, A. H., Tibben, A., van Ommen, G. J. B., and Roos, R. A. C. (2002). Behavioural complaints in participants who underwent predictive testing for Huntington's disease. J. Med. Genet. 39, 857–862. doi: 10.1136/jmg.39.11.857
World Health Organisation (2014). Diet, Nutrition and the Prevention of Chronic Diseases. WHO Tech. Rep. Ser. No. 916. Available online at: http://www.who.int/dietphysicalactivity/publications/trs916/summary/en/
Yang, Q., Khoury, M. J., Botto, L., Friedman, J. M., and Flanders, W. D. (2003). Improving the prediction of complex diseases by testing for multiple disease-susceptibility genes. Am. J. Hum. Genet. 72, 636–649. doi: 10.1086/367923
Yoon, P. (2005). CDC's family history public health initiative: 2005 update in Genomics and Population Health 2005, eds M. Gwinn, S. Bedrosian, D. Ottman, and M. J. Khoury (Atlanta, GA: Centers for Disease Control and Prevention, Office of Genomics and Disease Prevention), 11–13.
Keywords: genetic testing, genetic risk, chronic disease, psychological implication, quality of life, health psychology
Citation: Oliveri S, Ferrari F, Manfrinati A and Pravettoni G (2018) A Systematic Review of the Psychological Implications of Genetic Testing: A Comparative Analysis Among Cardiovascular, Neurodegenerative and Cancer Diseases. Front. Genet. 9:624. doi: 10.3389/fgene.2018.00624
Received: 17 October 2017; Accepted: 23 November 2018;
Published: 10 December 2018.
Edited by:
George P. Patrinos, University of Patras, GreeceReviewed by:
Evangelia Eirini Tsermpini, University of Patras, GreeceMathura Shanmugasundaram, Harvard Medical School, United States
Copyright © 2018 Oliveri, Ferrari, Manfrinati and Pravettoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serena Oliveri, serena.oliveri@unimi.it
 Serena Oliveri
Serena Oliveri Federica Ferrari
Federica Ferrari Andrea Manfrinati
Andrea Manfrinati Gabriella Pravettoni1,2
Gabriella Pravettoni1,2