-
PDF
- Split View
-
Views
-
Cite
Cite
Ajay Kumar, John H. Turney, Aleck M. Brownjohn, Michael J McMahon, Unusual bacterial infections of the urinary tract in diabetic patients—rare but frequently lethal, Nephrology Dialysis Transplantation, Volume 16, Issue 5, May 2001, Pages 1062–1065, https://doi.org/10.1093/ndt/16.5.1062
Close - Share Icon Share
(Section Editor: K. Kühn)
Case 1
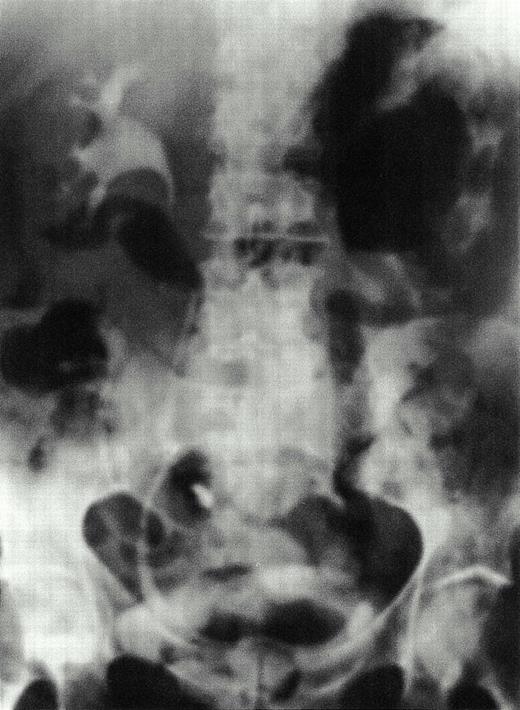
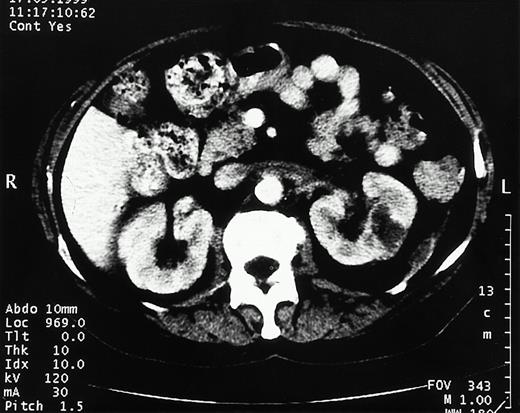
A 54‐year‐old, poorly compliant, insulin‐requiring, diabetic man, who was also known to have a history of alcohol abuse, was referred to hospital with a 10‐day history of progressive left‐sided abdominal and loin pain, dysuria and fever. The onset of pneumaturia had prompted him to seek medical advice. Physical examination revealed an obese, dehydrated, ill‐looking man with a temperature of 40°C. The blood pressure was 100/50 mmHg and pulse 105/min. He was markedly tender over the left side of the abdomen, and although bowel sounds were scanty there was no peritonism. Pertinent laboratory investigations showed a peripheral white cell count of 26×109/1, serum creatinine 185 μmol/l, urea 31 mmol/l and the glucose was 24 mmol/l. An IVU showed gas distending the left renal pelvis and ureter (Figure 1). Gas was also present in the renal parenchyma, extending into surrounding tissues, and was best seen on a CT scan (Figure 2). E. coli was isolated subsequently from both urine and blood cultures.
Within 6 h of admission to hospital, he developed features of septicaemic shock and was transferred to the intensive care unit. The patient was treated with cefotaxime and gentamicin, his fluid status was corrected, and noradrenaline and adrenaline were necessary to support his circulation. His condition deteriorated further, and tests revealed evidence of early disseminated intravascular coagulopathy. Despite the significant risks of surgery, a left nephrectomy was performed. Shortly after, he died from an anterior myocardial infarction.
Emphysematous pyelonephritis and pyelitis. IVU shows gas in the left renal parenchyma. Gas is also seen distending the left renal pelvis and outlining the ureter.
Emphysematous pyelonephritis. CT scan shows typical features of emphysematous pyelonephritis with loculation of gas and diffuse mottling of the parenchyma of the left kidney.
Case 2
A 55‐year‐old, insulin‐dependent, diabetic woman presented with a 2‐week history of unremitting right‐sided abdominal pain, dysuria and spiking fever. Previously, she had received many courses of antibiotics for recurrent urinary infections, but on this occasion, empirical antibiotic treatment prescribed by her family physician was unhelpful. She was taking a maintenance dose of prednisolone 7.5 mg daily to keep her in remission from both systemic lupus erythematosis and rheumatoid arthritis. She was febrile at 41°C and tender in the right flank of the abdomen. The white blood cell count was 22.6×109/l and the C‐reactive protein 340 mg/l (normal <10). Ultrasonography revealed a moderate degree of hydronephrosis of the right kidney and gas present in the upper ureter and pelvicalyceal system. Frank pus was obtained when a nephrostomy was inserted to relieve the urinary obstruction caused by a small calculus. Culture yielded a pure growth of Candida albicans. The emphysematous pyelitis cleared rapidly after treating the infection with fluconazole. This was given intravenously for the first 5 days before converting to an oral preparation of the drug and continued for another 2 weeks.
Case 3
A 66‐year‐old woman was referred to our hospital with ERCP‐induced necrotizing pancreatitis. Twelve days earlier, she had developed upper abdominal pain, vomiting and fever within a few hours of seemingly uneventful pancreatico‐biliary tract instrumentation to investigate mild biliary tract dilatation. The serum amylase was raised at 1838 IU/l (normal 0–100) and CT scan had shown a severely oedematous pancreas. Conservative measures including analgesia, antibiotics and intravenous fluids were unsuccessful, and repeat imaging 10 days later confirmed necrosis of the bulk of the pancreas, a perihepatic fluid collection and oedematous bowel. At laparotomy, almost the entire pancreas had necrosed and was resected, a large collection of retroperitoneal pus drained and a defunctioning loop ileostomy was created to conserve oedematous yet viable transverse colon.
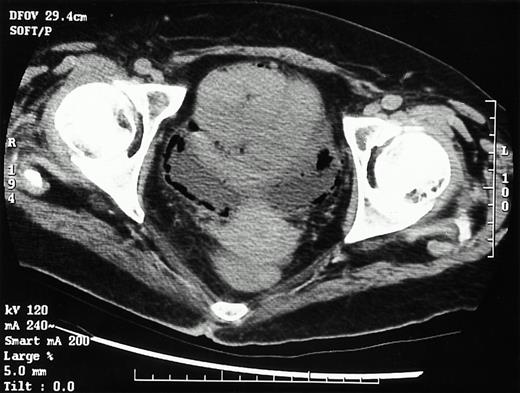
She made slow but steady progress but a few days after stopping antibiotic treatment, she complained of lower abdominal pain, the fever recurred, and glycaemic control became erratic. A CT scan of the lower abdomen and pelvis demonstrated intraluminal and intramural gas in the bladder caused by emphysematous cystitis (Figure 3). The patient had not noticed pneumaturia or haematuria although blood and protein were present on urine dipstix testing. E. coli was cultured in the urine and from blood cultures. A urinary bladder catheter that had been in situ for nearly 3 weeks was removed. This serious infection was treated successfully with ciprofloxacin given for a total of 3 weeks, the first 2 weeks by the intravenous route. Further assessment by ultrasound revealed no residual intramural gas.
Emphysematous cystitis. CT scan of the lower abdomen and pelvis demonstrating intraluminal and intramural gas in the bladder.
Discussion
Although gas‐producing infections account for a very small percentage of all bacterial infections of the urinary tract, they attract importance because of their life‐threatening potential [1–4]. The spectrum of gas‐ producing infections includes emphysematous pyelonephritis (case 1), emphysematous pyelitis (case 2), and emphysematous cystitis (case 3). These are three distinct clinical entities whose clinical course, management, and ultimate prognosis differ appreciably [2,5].
Emphysematous pyelonephritis is a severe, necrotizing infection with gas formation within the renal parenchyma and perirenal tissue. It should be considered in the differential diagnosis in any diabetic patient with unexplained sepsis, because over 90% of documented cases have occurred in diabetics [2]. Others at special risk of developing this infection include debilitated or immunocompromised individuals and those with pre‐existing renal impairment, especially ischaemic and diabetic nephropathy [2,3]. The clinical features of emphysematous pyelonephritis are indistinguishable from severe, acute pyelonephritis and the diagnosis may only be suspected after poor response to conventional therapy. The onset may be abrupt and devastating or the infection may run a protracted course with an average duration of symptoms of 18–21 days before diagnosis [1,3]. On the basis of clinical observations, CT scan appearances and histopathological findings, a modern classification of emphysematous pyelonephritis into types I and II, has emerged [6].
Type I emphysematous pyelonephritis runs a fulminant course with an overall mortality of 70% [6]. There is diffuse renal tissue destruction with severe inflammation progressing to widespread necrosis, intravascular thrombosis, microabscess and gas formation [3,7]. CT scan precisely defines areas of infarction and shows a streaky or diffuse mottling of the renal parenchyma, with gas distributed radially along the pyramids [6]. In about 50% of survivors, the affected kidney proves subsequently to be non‐functioning [3]. Type II emphysematous pyelonephritis follows a more indolent course with a lower death rate of approximately 20% [6]. Characteristic features on CT scan are renal and perirenal fluid collections with loculated gas. This correlates with histopathological features revealing a less aggressive inflammatory process, patchy rather than diffuse destruction of the renal parenchyma and localization of infection by abscess formation [6,7]. These differences suggest an intact, more appropriate host immune response and less compromise to the vascular supply in patients developing type II compared to those with type I infection.
Most infections are due to E. coli and Klebsiella species or mixed infections (20%) [2,3]. Less common gas‐producing bacterial species that have been cited include Proteus, Enterobacter, Streptococci, and Candida. Bacterial replication and fermentation is facilitated by a hyperglycaemic milieu in the context of impaired tissue perfusion or urinary obstruction [8]. Necrotic tissue then acts as a substrate to augment gas production [9,10]. Also, tissue ischaemia inhibits the dispersion of locally produced gas and prevents effective delivery of antibiotics to the site of infection [7]. Early, aggressive therapy is required to control diabetes, dehydration and the underlying infection. Subsequent management options should be based on the severity of the patient's condition, and the category of emphysematous pyelonephritis (type I or II), which is best established by CT scan appearance [6,11,12]. Other factors that influence outcome include age, pre‐existing renal impairment, palpable loin mass, crepitus, thrombocytopenia or other bleeding diathesis [13]. Accepted teaching is that nephrectomy or, where possible, open drainage is the treatment of choice for emphysematous pyelonephritis [3,4,7]. This combined medical and surgical approach reduces mortality to 20% compared to 80% mortality in those treated solely with antibiotics [2,3]. Overwhelming infection causing haemodynamic instability or a serious risk of bleeding denies surgery to the most seriously ill. Surgery is also an unattractive option for treating emphysematous pyelonephritis in a solitary kidney [14,15], or where there is bilateral renal involvement [16]. In such circumstances, percutaneous drainage under CT guidance offers the best chance of survival [17]. In expert hands, initial results suggest that this method can achieve similar results to surgical intervention.
Emphysematous pyelitis refers to infection that is restricted to the collecting system, sparing the renal parenchyma [2]. Correction of urinary obstruction and antimicrobial therapy usually leads to prompt resolution of the pyonephrosis. Nevertheless, there is a high mortality (20%), mainly because of failure to diagnose the condition before generalized sepsis and multisystem organ failure has intervened.
Emphysematous cystitis is characterized by pockets of gas formed in and around the bladder wall by fermenting organisms [18]. Various factors predispose to emphysematous cystitis including poorly controlled diabetes mellitus, recurrent urinary infections, indwelling urinary bladder catheters, bladder outlet obstruction, neurogenic bladder, and after kidney transplantation [14,19]. Emphysematous cystitis should be differentiated from other causes of air in the bladder, such as fistulous connections between the bladder and bowel or vagina, and air in the bladder lumen after trauma or instrumentation. Pneumaturia is a frequent finding [18], and other presenting symptoms are typical of any lower urinary tract infection—lower abdominal pain, fever, dysuria, frequency and urgency. E. coli is the commonest pathogen, but other species that have been implicated include Klebsiella, Proteus, Staphylococci, Streptococci, Enterobacter and Candida albicans [18,20]. Whereas plain radiography (including tomography) and ultrasonography are useful screening tools, a CT scan demonstrates the location and extent of the gas collection most reliably [11,12,21]. The prognosis for this condition is generally favourable, but there have been reports of severe necrotizing cystitis requiring cystectomy, and a mortality rate of 20% [18]. As with any gas‐producing infection, patient survival depends on early diagnosis with the correction of underlying causes, strict glycaemic control, a prolonged course of antibiotics (3–6 weeks), relief of obstruction to urinary outflow, and surgical excision where necessary.
Teaching point
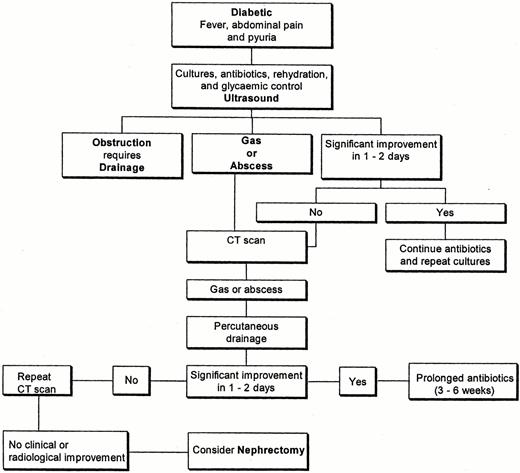
Gas‐producing or emphysematous infections of the urinary tract, which are observed mainly in diabetic patients, can be rapidly progressive and are frequently fatal. Diagnosis is often established late because of the non‐specific clinical features and infrequent occurrence of this condition. Delay in initiating appropriate treatment contributes to the high death rate, which, in some series, approaches 80%. Defining the precise location of the infection is important, since this has implications relating to management options and likely outcome. In managing gas‐forming infections of the renal tract, efforts to conserve the affected kidney can only be sustained if this does not compromise the patient's safety. A successful outcome can be achieved by early recognition of the infection, meticulous monitoring of progress by frequent clinical and sequential radiological assessment, and by timely surgical intervention when indicated (Figure 4).
Suggested algorithm for managing gas‐forming infections of the urinary tract.
Correspondence and offprint requests to: Dr A. Kumar, Senior Registrar in Renal Medicine, Department of Renal Medicine, The General Infirmary at Leeds, Great George Street, Leeds LS1 3EX, UK.
Supported by an educational grant from  Fresenius Medical Care
Fresenius Medical Care
References
Evanoff GV, Thompson CS, Foley R, Weinman EJ. Spectrum of gas within the kidney. Emphysematous pyelonephritis and emphysematous pyelitis.
Michaeli J, Mogle P, Perlberg S, Heiman S, Caine M. Emphysematous pyelonephritis.
Klein FA, Smith MJ, Vick CW, III, Schneider V. Emphysematous pyelonephritis: diagnosis and treatment.
Bohlman ME, Sweren BS, Khazan R, Minkin SD, Goldman SM, Fishman EK. Emphysematous pyelitis and emphysematous pyelonephritis characterized by computerized tomography.
Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas‐producing bacterial renal infection: correlation between imaging findings and clinical outcome.
Ahlering TE, Boyd SD, Hamilton CL, et al. Emphysematous pyelonephritis: a 5‐year experience with 13 patients.
Huang JJ, Chen KW, Ruaan MK. Mixed acid fermentation of glucose as a mechanism of emphysematous urinary tract infection.
Schainuck LI, Fouty R, Cutler RE. Emphysematous pyelonephritis. A new case and review of previous observations.
Yang WH, Shen NC. Gas‐forming infection of the urinary tract: an investigation of fermentation as a mechanism.
Goldman SM, Fishman EK. Upper urinary tract infection: the current role of CT, ultrasound, and MRI.
Joseph RC, Amendola MA, Artze ME et al. Genitourinary tract gas: imaging evaluation.
Wan YL, Lo SK, Bullard MJ, Chang PL, Lee TY. Predictors of outcome in emphysematous pyelonephritis.
Akalin E, Hyde C, Schmitt G, Kaufman J, Hamburger RJ. Emphysematous cystitis and pyelitis in a diabetic renal transplant recipient.
Godec CJ, Cass AS, Berkseth R. Emphysematous pyelonephritis in a solitary kidney.
Stein JP, Spitz A, Elmajian DA et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature.
Chen MT, Huang CN, Chou YH, Huang CH, Chiang CP, Liu GC. Percutaneous drainage in the treatment of emphysematous pyelonephritis: 10‐year experience.
Quint HJ, Drach GW, Rappaport WD, Hoffmann CJ. Emphysematous cystitis: a review of the spectrum of disease.
Kalra OP, Malik N, Minz M, Gupta KL, Sakhuja V, Chugh KS. Emphysematous pyelonephritis and cystitis in a renal transplant recipient—computed tomographic appearance.
Wortmann G, Fleckenstein J. Incidental discovery of emphysematous cystitis.





Comments