-
PDF
- Split View
-
Views
-
Cite
Cite
Stefano F. Rimoldi, Urs Scherrer, Franz H. Messerli, Secondary arterial hypertension: when, who, and how to screen?, European Heart Journal, Volume 35, Issue 19, 14 May 2014, Pages 1245–1254, https://doi.org/10.1093/eurheartj/eht534
Close - Share Icon Share
Abstract
Secondary hypertension refers to arterial hypertension due to an identifiable cause and affects ∼5–10% of the general hypertensive population. Because secondary forms are rare and work up is time-consuming and expensive, only patients with clinical suspicion should be screened. In recent years, some new aspects gained importance regarding this screening. In particular, increasing evidence suggests that 24 h ambulatory blood pressure (BP) monitoring plays a central role in the work up of patients with suspected secondary hypertension. Moreover, obstructive sleep apnoea has been identified as one of the most frequent causes. Finally, the introduction of catheter-based renal denervation for the treatment of patients with resistant hypertension has dramatically increased the interest and the number of patients evaluated for renal artery stenosis. We review the clinical clues of the most common causes of secondary hypertension. Specific recommendations are given as to evaluation and treatment of various forms of secondary hypertension. Despite appropriate therapy or even removal of the secondary cause, BP rarely ever returns to normal with long-term follow-up. Such residue hypertension indicates either that some patients with secondary hypertension also have concomitant essential hypertension or that irreversible vascular remodelling has taken place. Thus, in patients with potentially reversible causes of hypertension, early detection and treatment are important to minimize/prevent irreversible changes in the vasculature and target organs.
Introduction
Secondary hypertension is defined as increased systemic blood pressure (BP) due to an identifiable cause. Only 5–10% of patients suffering from arterial hypertension have a secondary form, whereas the vast majority has essential (idiopathic or primary) hypertension.1 Because secondary forms are rare and screening for them expensive and laborious, it is not cost effective to search for secondary causes of hypertension in every patient.
Moreover, while the majority of young patients (<40 years old) with secondary hypertension respond to specific treatment, in >35% of elderly patients target BP values are not achieved even after specific treatment.2 This suggests that on the one side, early detection and treatment of secondary hypertension are important to minimize irreversible changes in the systemic vasculature,3 on the other side, the prevalence of concomitant primary and secondary hypertension rises with increasing age.2
The aim of this review is to give indications which hypertensive patients should be screened for secondary forms and the tests to be used.
Prevalence
The prevalence of secondary hypertension depends mostly on age and clinical characteristics of the screened population.4–7 We should remember that ‘essential’ hypertension is not less common in patients who have secondary hypertension than in the general population. Thus, not uncommonly there remains some residue hypertension after the pathogenetic cause of secondary hypertension has been identified and removed. In patients with resistant hypertension, defined as increased BP despite the use of three antihypertensive drugs including a diuretic at the optimal dosages, the prevalence of secondary forms is significantly higher than in patients with controlled BP (Table 1).8
Overview of the most common causes for secondary hypertension
| Secondary cause . | Prevalencea . | Prevalenceb . | History . | Screening . | Clinical findings . | Laboratory findings . |
|---|---|---|---|---|---|---|
| Obstructive sleep apnoea | >5–15% | >30% | Snoring, daytime sleepiness, morning headache, irritability | Screening questionnaire; polysomnography | ↑ neck circumference; obesity; peripheral oedema | Not specific |
| Renal parenchymal disease | 1.6–8.0% | 2–10% | Loss of good BP-control; diabetes; smoking; generalized atherosclerosis; previous renal failure; nocturia | Creatinine, ultrasound of the kidney | Peripheral oedema; pallor; loss of muscle mass | ↑ Creatinine, proteinuria; ↓ Ca2+, ↑ K+, ↑ PO4 |
| Renal artery stenosis | 1.0–8.0% | 2.5–20% | Generalized atherosclerosis; diabetes; smoking; recurrent flush pulmonary oedema | Duplex, or CT, or MRI, or angiography (drive by) | Abdominal bruits; peripheral vascular disease; | Secondary aldosteronism: ARR →; ↓ K+; ↓ Na+ |
| Primary aldosteronism | 1.4–10% | 6–23% | Fatigue; constipation; polyuria, polydipsia | Aldosterone–renin ratio (ARR) | Muscle weakness | ↓ K+; ARR ↑ |
| Thyroid disease | 1–2% | 1–3% | Hyperthyreoidism; palpitations, weight loss, anxiety, heat intolerance; Hypothyreodism; weight gain, fatigue, obstipation | TSH | Hyperthyreodism: tachycardia, AF; accentuated heart sounds; exophthalmus; Hypothyreodism; Bradycardia; muscle weakness; myxoedema | Hyperthyreodism: TSH↓; fT4 and/or fT3 ↑; Hypothyreodism: TSH ↑; fT4 ↓; cholesterol ↑ |
| Cushing's Syndrome | 0.5% | <1.0% | Weight gain; impotence; fatigue; psychological changes; polydypsia and polyuria | 24 h urinary cortisol; dexamethasone testing | Obesity, hirsutism, skin atrophy, Striae rubrae, muscle weakness, osteopenia | 24 h urinary; cortisol ↑; Glucose↑; Cholesterol ↑; K+↓ |
| Phaeochromocytoma | 0.2–0.5% | <1% | Headache; palpitations; flushing; anxiety | Plasma-metanephrines; 24 h urinary catecholamine | The 5 ‘Ps’c: paroxysmal hypertension; pounding headache; perspiration; palpitations; pallor | metanephrines ↑ |
| Coarctation of the aorta | <1% | <1% | Headache; nose bleeding; leg weakness or claudicatio | Cardiac ultrasound | Different BP (≥20/10 mmHg) between upper–lower extremities and/or between right–left arm; ↓ and delayed femoral pulsations; interscapular ejection murmur; rib notching on chest Rx | Not specific |
| Secondary cause . | Prevalencea . | Prevalenceb . | History . | Screening . | Clinical findings . | Laboratory findings . |
|---|---|---|---|---|---|---|
| Obstructive sleep apnoea | >5–15% | >30% | Snoring, daytime sleepiness, morning headache, irritability | Screening questionnaire; polysomnography | ↑ neck circumference; obesity; peripheral oedema | Not specific |
| Renal parenchymal disease | 1.6–8.0% | 2–10% | Loss of good BP-control; diabetes; smoking; generalized atherosclerosis; previous renal failure; nocturia | Creatinine, ultrasound of the kidney | Peripheral oedema; pallor; loss of muscle mass | ↑ Creatinine, proteinuria; ↓ Ca2+, ↑ K+, ↑ PO4 |
| Renal artery stenosis | 1.0–8.0% | 2.5–20% | Generalized atherosclerosis; diabetes; smoking; recurrent flush pulmonary oedema | Duplex, or CT, or MRI, or angiography (drive by) | Abdominal bruits; peripheral vascular disease; | Secondary aldosteronism: ARR →; ↓ K+; ↓ Na+ |
| Primary aldosteronism | 1.4–10% | 6–23% | Fatigue; constipation; polyuria, polydipsia | Aldosterone–renin ratio (ARR) | Muscle weakness | ↓ K+; ARR ↑ |
| Thyroid disease | 1–2% | 1–3% | Hyperthyreoidism; palpitations, weight loss, anxiety, heat intolerance; Hypothyreodism; weight gain, fatigue, obstipation | TSH | Hyperthyreodism: tachycardia, AF; accentuated heart sounds; exophthalmus; Hypothyreodism; Bradycardia; muscle weakness; myxoedema | Hyperthyreodism: TSH↓; fT4 and/or fT3 ↑; Hypothyreodism: TSH ↑; fT4 ↓; cholesterol ↑ |
| Cushing's Syndrome | 0.5% | <1.0% | Weight gain; impotence; fatigue; psychological changes; polydypsia and polyuria | 24 h urinary cortisol; dexamethasone testing | Obesity, hirsutism, skin atrophy, Striae rubrae, muscle weakness, osteopenia | 24 h urinary; cortisol ↑; Glucose↑; Cholesterol ↑; K+↓ |
| Phaeochromocytoma | 0.2–0.5% | <1% | Headache; palpitations; flushing; anxiety | Plasma-metanephrines; 24 h urinary catecholamine | The 5 ‘Ps’c: paroxysmal hypertension; pounding headache; perspiration; palpitations; pallor | metanephrines ↑ |
| Coarctation of the aorta | <1% | <1% | Headache; nose bleeding; leg weakness or claudicatio | Cardiac ultrasound | Different BP (≥20/10 mmHg) between upper–lower extremities and/or between right–left arm; ↓ and delayed femoral pulsations; interscapular ejection murmur; rib notching on chest Rx | Not specific |
BP, blood pressure; Ca2+, calcium; K+, potassium; PO4, phosphate; CT, computer tomography; ARR, aldosterone–renin ratio; Na+, sodium; AF, atrial fibrillation; TSH, thyroid-stimulating hormone; fT4, free thyroxine; fT3, free triiodothyronine.
aPrevalence in hypertensive patients.
bPrevalence in patients with resistant hypertension.
cKaplan's, Clinical hypertension, Tenth Edition, 2010, Lippincott Williams & Wilkins, p. 363.
Overview of the most common causes for secondary hypertension
| Secondary cause . | Prevalencea . | Prevalenceb . | History . | Screening . | Clinical findings . | Laboratory findings . |
|---|---|---|---|---|---|---|
| Obstructive sleep apnoea | >5–15% | >30% | Snoring, daytime sleepiness, morning headache, irritability | Screening questionnaire; polysomnography | ↑ neck circumference; obesity; peripheral oedema | Not specific |
| Renal parenchymal disease | 1.6–8.0% | 2–10% | Loss of good BP-control; diabetes; smoking; generalized atherosclerosis; previous renal failure; nocturia | Creatinine, ultrasound of the kidney | Peripheral oedema; pallor; loss of muscle mass | ↑ Creatinine, proteinuria; ↓ Ca2+, ↑ K+, ↑ PO4 |
| Renal artery stenosis | 1.0–8.0% | 2.5–20% | Generalized atherosclerosis; diabetes; smoking; recurrent flush pulmonary oedema | Duplex, or CT, or MRI, or angiography (drive by) | Abdominal bruits; peripheral vascular disease; | Secondary aldosteronism: ARR →; ↓ K+; ↓ Na+ |
| Primary aldosteronism | 1.4–10% | 6–23% | Fatigue; constipation; polyuria, polydipsia | Aldosterone–renin ratio (ARR) | Muscle weakness | ↓ K+; ARR ↑ |
| Thyroid disease | 1–2% | 1–3% | Hyperthyreoidism; palpitations, weight loss, anxiety, heat intolerance; Hypothyreodism; weight gain, fatigue, obstipation | TSH | Hyperthyreodism: tachycardia, AF; accentuated heart sounds; exophthalmus; Hypothyreodism; Bradycardia; muscle weakness; myxoedema | Hyperthyreodism: TSH↓; fT4 and/or fT3 ↑; Hypothyreodism: TSH ↑; fT4 ↓; cholesterol ↑ |
| Cushing's Syndrome | 0.5% | <1.0% | Weight gain; impotence; fatigue; psychological changes; polydypsia and polyuria | 24 h urinary cortisol; dexamethasone testing | Obesity, hirsutism, skin atrophy, Striae rubrae, muscle weakness, osteopenia | 24 h urinary; cortisol ↑; Glucose↑; Cholesterol ↑; K+↓ |
| Phaeochromocytoma | 0.2–0.5% | <1% | Headache; palpitations; flushing; anxiety | Plasma-metanephrines; 24 h urinary catecholamine | The 5 ‘Ps’c: paroxysmal hypertension; pounding headache; perspiration; palpitations; pallor | metanephrines ↑ |
| Coarctation of the aorta | <1% | <1% | Headache; nose bleeding; leg weakness or claudicatio | Cardiac ultrasound | Different BP (≥20/10 mmHg) between upper–lower extremities and/or between right–left arm; ↓ and delayed femoral pulsations; interscapular ejection murmur; rib notching on chest Rx | Not specific |
| Secondary cause . | Prevalencea . | Prevalenceb . | History . | Screening . | Clinical findings . | Laboratory findings . |
|---|---|---|---|---|---|---|
| Obstructive sleep apnoea | >5–15% | >30% | Snoring, daytime sleepiness, morning headache, irritability | Screening questionnaire; polysomnography | ↑ neck circumference; obesity; peripheral oedema | Not specific |
| Renal parenchymal disease | 1.6–8.0% | 2–10% | Loss of good BP-control; diabetes; smoking; generalized atherosclerosis; previous renal failure; nocturia | Creatinine, ultrasound of the kidney | Peripheral oedema; pallor; loss of muscle mass | ↑ Creatinine, proteinuria; ↓ Ca2+, ↑ K+, ↑ PO4 |
| Renal artery stenosis | 1.0–8.0% | 2.5–20% | Generalized atherosclerosis; diabetes; smoking; recurrent flush pulmonary oedema | Duplex, or CT, or MRI, or angiography (drive by) | Abdominal bruits; peripheral vascular disease; | Secondary aldosteronism: ARR →; ↓ K+; ↓ Na+ |
| Primary aldosteronism | 1.4–10% | 6–23% | Fatigue; constipation; polyuria, polydipsia | Aldosterone–renin ratio (ARR) | Muscle weakness | ↓ K+; ARR ↑ |
| Thyroid disease | 1–2% | 1–3% | Hyperthyreoidism; palpitations, weight loss, anxiety, heat intolerance; Hypothyreodism; weight gain, fatigue, obstipation | TSH | Hyperthyreodism: tachycardia, AF; accentuated heart sounds; exophthalmus; Hypothyreodism; Bradycardia; muscle weakness; myxoedema | Hyperthyreodism: TSH↓; fT4 and/or fT3 ↑; Hypothyreodism: TSH ↑; fT4 ↓; cholesterol ↑ |
| Cushing's Syndrome | 0.5% | <1.0% | Weight gain; impotence; fatigue; psychological changes; polydypsia and polyuria | 24 h urinary cortisol; dexamethasone testing | Obesity, hirsutism, skin atrophy, Striae rubrae, muscle weakness, osteopenia | 24 h urinary; cortisol ↑; Glucose↑; Cholesterol ↑; K+↓ |
| Phaeochromocytoma | 0.2–0.5% | <1% | Headache; palpitations; flushing; anxiety | Plasma-metanephrines; 24 h urinary catecholamine | The 5 ‘Ps’c: paroxysmal hypertension; pounding headache; perspiration; palpitations; pallor | metanephrines ↑ |
| Coarctation of the aorta | <1% | <1% | Headache; nose bleeding; leg weakness or claudicatio | Cardiac ultrasound | Different BP (≥20/10 mmHg) between upper–lower extremities and/or between right–left arm; ↓ and delayed femoral pulsations; interscapular ejection murmur; rib notching on chest Rx | Not specific |
BP, blood pressure; Ca2+, calcium; K+, potassium; PO4, phosphate; CT, computer tomography; ARR, aldosterone–renin ratio; Na+, sodium; AF, atrial fibrillation; TSH, thyroid-stimulating hormone; fT4, free thyroxine; fT3, free triiodothyronine.
aPrevalence in hypertensive patients.
bPrevalence in patients with resistant hypertension.
cKaplan's, Clinical hypertension, Tenth Edition, 2010, Lippincott Williams & Wilkins, p. 363.
In children and adolescents, the most common causes for hypertension are renal parenchymal or vascular disease and aortic coarctation.9 In adults, earlier studies identified renal parenchymal and vascular disease as the most common causes of secondary hypertension. More recently, obstructive sleep apnoea (OSA) was recognized as an exceedingly common cause of secondary hypertension (Table 1).10 Of the endocrine causes associated with hypertension, primary aldosteronism is the most common, followed by thyroid disease (hypo- or hyperthyreoidism), hypercortisolism (Cushing's), and finally phaeochromocytoma.
Who should be screened?
General clinical characteristics suggestive of secondary hypertension
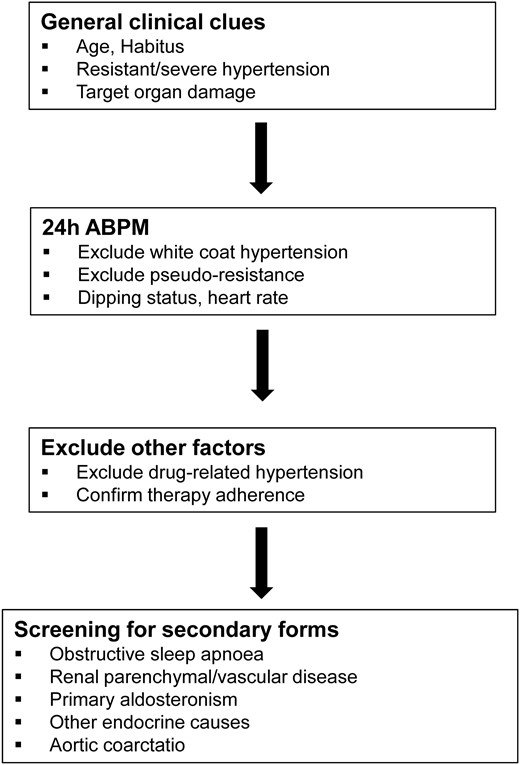
At the initial evaluation of a hypertensive patient, it is important to identify some general clinical clues that could suggest the presence of a secondary form of hypertension (Table 2; Figure 1).
General clinical characteristics suggestive of secondary hypertension
| Early onset of hypertension (i.e. <30 years) in patients without other risk factors (i.e. family history, obesity, etc.); increased BP in prepubertal children |
| Resistant hypertension (>140/90 mmHg despite three antihypertensive drugs including a diuretic) |
| Severe hypertension (>180/110 mmHg) or hypertensive emergencies |
| Sudden increase of BP in a previously stable patient |
| Non-dipping or reverse dipping during 24 h ambulatory BP monitoring |
| Presence of target organ damage (i.e. LVH, hypertensive retinopathy, etc.) |
| Early onset of hypertension (i.e. <30 years) in patients without other risk factors (i.e. family history, obesity, etc.); increased BP in prepubertal children |
| Resistant hypertension (>140/90 mmHg despite three antihypertensive drugs including a diuretic) |
| Severe hypertension (>180/110 mmHg) or hypertensive emergencies |
| Sudden increase of BP in a previously stable patient |
| Non-dipping or reverse dipping during 24 h ambulatory BP monitoring |
| Presence of target organ damage (i.e. LVH, hypertensive retinopathy, etc.) |
BP, blood pressure; LVH, left ventricular hypertrophy.
General clinical characteristics suggestive of secondary hypertension
| Early onset of hypertension (i.e. <30 years) in patients without other risk factors (i.e. family history, obesity, etc.); increased BP in prepubertal children |
| Resistant hypertension (>140/90 mmHg despite three antihypertensive drugs including a diuretic) |
| Severe hypertension (>180/110 mmHg) or hypertensive emergencies |
| Sudden increase of BP in a previously stable patient |
| Non-dipping or reverse dipping during 24 h ambulatory BP monitoring |
| Presence of target organ damage (i.e. LVH, hypertensive retinopathy, etc.) |
| Early onset of hypertension (i.e. <30 years) in patients without other risk factors (i.e. family history, obesity, etc.); increased BP in prepubertal children |
| Resistant hypertension (>140/90 mmHg despite three antihypertensive drugs including a diuretic) |
| Severe hypertension (>180/110 mmHg) or hypertensive emergencies |
| Sudden increase of BP in a previously stable patient |
| Non-dipping or reverse dipping during 24 h ambulatory BP monitoring |
| Presence of target organ damage (i.e. LVH, hypertensive retinopathy, etc.) |
BP, blood pressure; LVH, left ventricular hypertrophy.
Work-up in patients with suspected secondary hypertension. 24 h-ABPM, 24 h ambulatory blood-pressure monitoring.
Age
The most common causes of hypertension in prepubertal children are renal parenchymal or vascular disease and aortic coarctation.9 Young adults (<30 years) without a family history or other risk factors for hypertension should undergo screening for secondary forms. In elderly adults with known atherosclerosis, the presence of severe hypertension or an acute increase of BP is suggestive for a secondary form [i.e. renal artery stenosis (RAS)].
Habitus
Overweight patients with resistant hypertension should be screened for OSA (see below) and endocrine forms of hypertension (i.e. Cushing, hypothyroidism).
Blood pressure
Patients with resistant hypertension despite adequate drug therapy, severe hypertension at presentation (>180/110 mmHg), or hypertensive emergencies5 should be screened for a secondary form. The absence of a nighttime drop in BP (‘dipping’) of >10% relative to the daytime value during ambulatory BP monitoring (ABPM) was found to be associated with several secondary forms of hypertension (i.e. OSA, RAS, etc.).11,12 Therefore non-dippers (or even more so reverse nocturnal dippers) should undergo screening for secondary forms.
Generalized atherosclerosis
Roughly 15–30% of patients with arterial hypertension and diffuse atherosclerotic disease (i.e. coronary, peripheral, cerebrovascular disease) have a significant RAS (defined as ≥50%).13,14 The presence of resistant hypertension, the sudden increase of a previously well-controlled arterial hypertension or non-dipping are all suggestive of a relevant RAS.
‘Second diagnostic look’ after starting antihypertensive therapy
There are some situations that may suggest the presence of a secondary hypertension after starting antihypertensive therapy. A second diagnostic look is indicated if there is:
an excessive drop in potassium with a small dose of a diuretic (primary aldosteronism or other endogenous or exogenous mineralocorticoid excess)
Excessive decrease in GFR with a small dose of an ACE-inhibitor (RAS, predominately when bilateral)
a remarkably resistant arterial hypertension
blood pressure decreases with treatment but remains excessively labile
What to do before screening: exclude pseudo-hypertension and pseudo-resistance
Patients with resistant arterial hypertension should be screened for secondary forms.8 However, before starting the screening, pseudo-hypertension and pseudo-resistance should be excluded.8
Pseudo-hypertension
Pseudo-hypertension is defined as cuff diastolic BP at least 15 mmHg higher than simultaneously measured intra-arterial BP. It is found in elderly patients with calcified and rigid arteries who present with little or no target organ damage despite very high BP readings. In these patients, excessive cuff pressure is needed to compress the artery resulting in falsely high readings. This entity may be identified using the Osler manoeuvre.15 However, this manoeuvre has a large intra- and inter-observer variability.16 Assessment of brachial arterial stiffness by measurement of carotid-radial pulse wave velocity may also help identify patients with this particular form of hypertension.16
Pseudo-resistance: inadequate blood pressure measurement techniques
There are two very common causes for pseudo-resistance due to inaccurate BP measurement techniques: (i) BP measurement without letting the patient quietly sit for at least 5 min and (ii) inadequate cuff size (too small cuff size may result in falsely increased BP >15 mmHg).17
Poor treatment control
Poor therapy control is sometimes related to physician's ‘clinical inertia’, i.e. they fail to up titrate therapy in order to reach target BP. Conversely, poor patient adherence to therapy is widespread reaching about 40% in patients with newly diagnosed hypertension.18
White coat hypertension
White coat hypertension is a frequent cause of pseudo-resistance with a prevalence of about 20–30%.19 Twenty-four hour ABPM is a valuable tool to assess the likelihood of secondary hypertension.
Drug-related hypertension
Several medications are related to treatment resistance.20 The most common are NSAIDs and glucocorticoids probably via sodium and fluid retention particularly in patients with kidney disease. NSAIDs may increase mean 24 h systolic BP by 4–5 mmHg, particularly in patients with pre-existing hypertension and in salt-sensitive patients.21 Therefore, in patients with hypertension, acetaminophen is recommended as analgesic of choice. However, a very recent study showed that even treatment with acetaminophen was associated with increased 24 h systolic and diastolic BP (2.9 and 2.2 mmHg, respectively, vs. placebo) in patients with coronary artery disease.22
Diet pills (i.e. phenylpropanolamine and sibutramine), stimulants (i.e. amphetamines and cocaine), and decongestants (i.e. phenylephrine hydrochloride and naphazoline hydrochloride) increase BP via activation of the sympathetic nervous system; to note, cocaine use is associated with acute, but not chronic hypertension. Licorice increases BP via stimulation of the mineralocorticoid receptor and inhibition of cortisol metabolism.23
Oral contraceptives (oestrogen + progestin) induce hypertension in about 5% of women.24 The increase in usually small, however, severe hypertensive episodes may occur.
Antidepressant agents (i.e. venlaflaxine and monoamine oxidase inhibitors) increase BP in a dose-dependent manner, probably via noradrenergic stimulation.
Immunosuppressive agents, particularly cyclosporine A, increase BP by sympathetic activation25 and direct vasoconstriction. Tacrolimus has less and rapamycin has almost no effect on BP.
Inhibitors of vascular endothelial growth factor (VEGFi) (i.e. bevacizumab, Avastin®) or tyrosine kinase inhibitors (i.e. sunitinib, Sutent®, sorafenib, Nexavar®) have been reported to increase BP. This adverse effect has to be considered a class-adverse effect of anti-angiogenic drugs. Mechanisms involved in the development of hypertension include decrease in nitric oxide bioavailability, rarefaction of the microvascular bed, and activation of endothelin-1 synthesis, one of the most potent vasoconstrictors.26,27
With Bevacizumab (Avastin®), the first drug of this class introduced in 2004 for the treatment of colorectal cancer, breast cancer, and renal cell carcinoma, the incidence of hypertension is dose related. Patients treated with bevacizumab may have a five-fold higher incidence of severe hypertension (i.e. >200/100 mmHg) compared with placebo.28,29 After 6 months of treatment with bevacizumab, SBP increased from 129 to 145 mmHg and in DBP from 75 to 82 mmHg.27
In a recent meta-analysis, the incidence of hypertension and severe hypertension in patients receiving sunitinib (Sutent®) was 21.6 and 6.8%, respectively.30 In the same meta-analysis, treatment with Sorafenib (Nexavar®) was associated with an incidence of hypertension and severe hypertension of 23.4 and 5.7%, respectively.30
Hypertension associated with VEGFi is often transient and resolves with discontinuation of the drug. There are no data available to recommend a specific first-line antihypertensive drug in this situation.
Role of ambulatory 24 h blood pressure monitoring
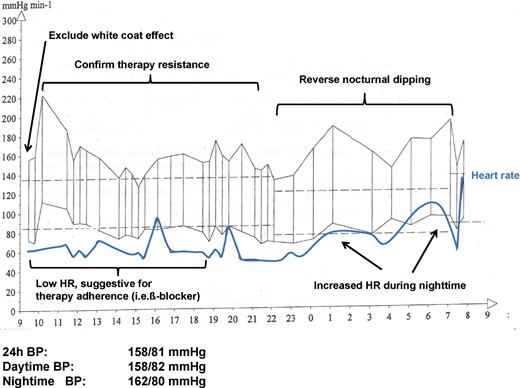
Ambulatory BP monitoring is the best measurement method to assess arterial BP.12 Ambulatory BP monitoring has a central role if a secondary form of arterial hypertension is suspected since it allows to exclude white coat hypertension, assess treatment-adherence, confirm the presence of resistant hypertension, and assess the dipping status (Figure 2). While reference values for office BP measurements aim to distinguish between normotensive and hypertensive subjects and are therefore ‘diagnostic’ cut-off values, reference values for 24 h ABPM in adults have been defined as outcome-driven thresholds and are considerably lower than clinical ones (Table 3).1,31,32 In children and adolescents, thresholds are based on population distribution.32
Thresholds values for office, home and ambulatory blood pressure measurement
| Category . | Systolic (mmHg) . | Diastolic (mmHg) . |
|---|---|---|
| Office BP | ≥140 | ≥90 |
| Home BP | ≥135 | ≥85 |
| Ambulatory BP | ||
| 24 h | ≥130 | ≥80 |
| Daytime (or awake) | ≥135 | ≥85 |
| Nighttime (or asleep) | ≥120 | ≥70 |
| Category . | Systolic (mmHg) . | Diastolic (mmHg) . |
|---|---|---|
| Office BP | ≥140 | ≥90 |
| Home BP | ≥135 | ≥85 |
| Ambulatory BP | ||
| 24 h | ≥130 | ≥80 |
| Daytime (or awake) | ≥135 | ≥85 |
| Nighttime (or asleep) | ≥120 | ≥70 |
Thresholds values for office, home and ambulatory blood pressure measurement
| Category . | Systolic (mmHg) . | Diastolic (mmHg) . |
|---|---|---|
| Office BP | ≥140 | ≥90 |
| Home BP | ≥135 | ≥85 |
| Ambulatory BP | ||
| 24 h | ≥130 | ≥80 |
| Daytime (or awake) | ≥135 | ≥85 |
| Nighttime (or asleep) | ≥120 | ≥70 |
| Category . | Systolic (mmHg) . | Diastolic (mmHg) . |
|---|---|---|
| Office BP | ≥140 | ≥90 |
| Home BP | ≥135 | ≥85 |
| Ambulatory BP | ||
| 24 h | ≥130 | ≥80 |
| Daytime (or awake) | ≥135 | ≥85 |
| Nighttime (or asleep) | ≥120 | ≥70 |
Ambulatory 24 h blood pressure (BP) monitoring in a patient with newly diagnosed obstructive sleep apnoea. Note the reverse nocturnal dipping and the increased heart rate (HR) during nighttime, possibly due to exaggerated sympathetic nervous system activity.
The presence of reverse nocturnal dipping eventually associated with increased heart rate is suggestive of the presence of a secondary form (i.e. OSA, RAS). Of note, sleep deprivation by cuff inflation during nocturnal BP monitoring may result in impaired or even reverse dipping.33
Role of echocardiography
Echocardiography is an important diagnostic tool in patients with (suspected) secondary hypertension. In particular, the presence of left ventricular hypertrophy (LVH) disproportionate to the duration of hypertension may suggest secondary hypertension (i.e. primary aldosteronism or renovascular hypertension).3,34 In patients with OSA, LVH is a very common finding and may be accompanied by left atrial enlargement and right ventricular hypertrophy.35 Finally, in young adults with hypertension, echocardiography is the screening method of choice for coarctation of the aorta (see below).
How to screen
In the following, we will describe the screening for the most common causes of secondary hypertension (Table 1).
Common causes of secondary hypertension
Obstructive sleep apnoea
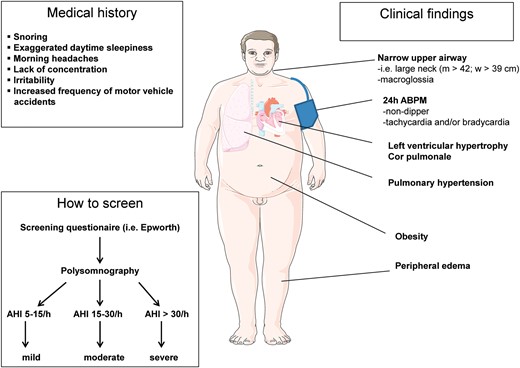
Obstructive sleep apnoea has been recognized as one of the most common causes for secondary hypertension (Figure 3).10,36 It is characterized by recurrent obstructive apnoeas and hypopnoeas caused by collapse of the upper airways during sleep. The severity of OSA is classified based on the apnoea–hypopnoea index [AHI, number of apnoeas plus hypopnoeas per hour of sleep: mild (AHI 5–15), moderate (AHI 16–30), and severe (AHI > 30)].37 Most patients with OSA complain of exaggerated daytime sleepiness, snoring, morning headache, lack of concentration, and irritability. Typical clinical findings are obesity, large neck, and macroglossia. Both nocturnal (non-dipping) and daytime BP are increased. Patients often display significant tachycardia and/or bradycardia during nighttime (Figure 2), probably due to increased sympathetic nerve activity and respectively increased vagal tone.38
Medical history, clinical findings, and screening work-up in patients with suspected obstructive sleep apnoea. AHI, apnoea-hypopnoea-index; 24 h-ABPM, 24 h ambulatory blood-pressure monitoring.
Mechanisms proposed to explain the BP elevation in OSA are increased sympathetic nerve activity39,40 and alterations of the renin–angiotensin–aldosterone system41,42 as a result of recurrent nocturnal hypoxemia. Moreover, hypoxemia has been associated with systemic endothelial dysfunction43,44 even in the absence of traditional CV risk factors,45 possibly mediated by excessive oxidative stress.46,47 Several studies have shown a decrease in nocturnal48 or in both nocturnal and daytime BP49–51 after successful continuous positive airway pressure therapy of OSA.
A history of snoring and daytime sleepiness should prompt to suspect OSA and assess sleepiness using the Epworth Sleepiness scale.52,53 In this questionnaire, patients have to rate their probability to fall asleep during eight different situations of daily life [between 0 (would never doze) to 3 (high chance of dozing)]. Patients with an Epworth score ≥10 (indicating the presence of excessive daytime sleepiness) and a high clinical suspicion of OSA need to be assessed by polysomnography.54 We recommend that patients with OSA undergo echocardiography to assess systolic left and right ventricular function, left ventricular mass, and estimate pulmonary artery pressure.
Renal parenchymal disease
Renal parenchymal disease is the most common cause of secondary hypertension in children9 and the second most common cause in adults.55 Urine analysis (protein, erythrocytes, and leucocytes) and measurement of serum creatinine concentration are the best screening methods. If creatinine concentration and/or urine analysis are pathologic, a renal ultrasound should be the next step.
Renal artery stenosis
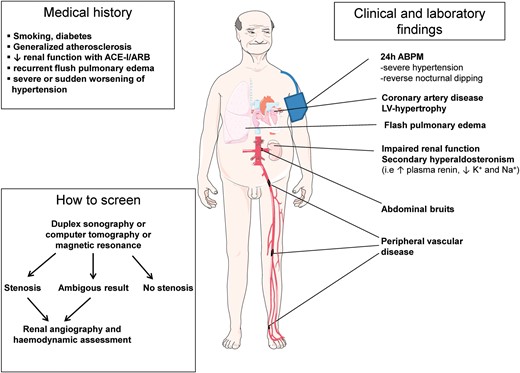
In children and young adults, fibromuscular dysplasia of the renal artery is one of the most common causes of secondary hypertension and should be excluded by imaging (screening with duplex ultrasound, confirmation by angiography) (Figure 4). If fibromuscular dysplasia is detected, other vascular beds (i.e. cerebrovascular) should be screened.56
Medical history, clinical findings, and screening work-up in patients with suspected atherosclerotic renal artery stenosis. ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; 24 h-ABPM, 24 h ambulatory blood-pressure monitoring; K+, potassium; Na+, sodium.
In adults, the most common form of renovascular disease is atherosclerotic RAS.13 The prevalence of RAS in a general population of hypertensive patients is between 1 and 8%,57,58 whereas in patients with generalized atherosclerosis its prevalence can be as high as 25–35%.59 Other clinical clues pointing to RAS in adult patients are: an abdominal bruit, particularly when diastolic; deterioration of the renal function with angiotensin converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers; severe or sudden worsening of hypertension in smokers or in patients with diabetes; diffuse atherosclerosis and recurrent flash pulmonary oedema.60,61 Further work up if indicated (Table 4) includes imaging by duplex-ultrasound, CT, or MRI.62 If screening confirms significant RAS renal angiography with haemodynamic assessment to detect a significant translesional gradient should be considered.63
Factors predicting effective revascularization of renal artery stenosis
| Resistant hypertension |
| Recent onset/progression of (severe) hypertension |
| Recent renal function deterioration |
| Acute renal function deterioration during therapy with ACE-I or ARB |
| Flash pulmonary oedema |
| Resistant hypertension |
| Recent onset/progression of (severe) hypertension |
| Recent renal function deterioration |
| Acute renal function deterioration during therapy with ACE-I or ARB |
| Flash pulmonary oedema |
RAS, renal artery stenosis; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin-receptor blocker.
Factors predicting effective revascularization of renal artery stenosis
| Resistant hypertension |
| Recent onset/progression of (severe) hypertension |
| Recent renal function deterioration |
| Acute renal function deterioration during therapy with ACE-I or ARB |
| Flash pulmonary oedema |
| Resistant hypertension |
| Recent onset/progression of (severe) hypertension |
| Recent renal function deterioration |
| Acute renal function deterioration during therapy with ACE-I or ARB |
| Flash pulmonary oedema |
RAS, renal artery stenosis; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin-receptor blocker.
After percutaneous (or surgical) treatment of RAS, as long as BP and renal function (GFR) remain stable and well controlled, no further exams are necessary. Otherwise, renal perfusion/vessel patency should be assessed with duplex ultrasonography or MRI.64
Atherosclerotic renal artery stenosis: to screen or not to screen?
Despite substantial improvement in imaging techniques over the past decade, clinicians have become increasingly reluctant to search for RAS. A reason for abandoning the diagnostic trajectory is doubt as to whether treatment of atherosclerotic RAS with angioplasty confers any benefit to the patient. Several studies have shown that rates of renal events, major cardiovascular events, and death were similar in the group assigned to undergo revascularization compared with the control group that received medical treatment only.65 Moreover, atherosclerotic RAS may be a complication of a pre-existent hypertension without a significant contribution to the increased BP values, and there are no tests with sufficient specificity and sensitivity to assess the contribution of the stenosis on BP. Its contribution can only be assessed post hoc, i.e. when arterial BP is decreased or normalized after revascularization. We thoroughly agree with the observation of De Leeuw et al.66 that the search for RAS in patients with hypertension is most often an exercise in futility since stenting of atherosclerotic RAS confers little benefit.65 A patient with longstanding hypertension, an estimated GFR of 30 mL/min, and contralateral nephrosclerosis is very unlikely to benefit from revascularization. However, an entity that needs to be considered in this context is the Pickering Syndrome being defined as flash pulmonary oedema secondary to bilateral RAS.60,61 Clearly in this situation, urgent revascularization will prove to be lifesaving. Thus practicing physicians cannot simply ‘forget’ about RAS despite the fact that randomized trials showed little if any benefit of revascularization in patients with longstanding disease. Finally, the recent introduction of catheter-based renal denervation for the treatment of patients with resistant hypertension has dramatically increased the interest and the number of patients evaluated for RAS. At this time, there is insufficient data on the long-term effects of renal denervation on arterial BP and it remains to be seen how this new technique will change the approach to resistant hypertension and RAS.67
Primary aldosteronism
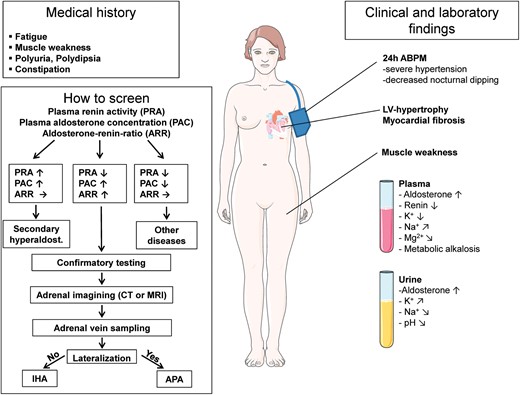
Primary (hyper)aldosteronism (PA), or Conn-Syndrome, refers to inappropriately high aldosterone synthesis that is independent of the renin–angiotensin system and cannot be suppressed by sodium loading (Figure 5). It is characterized by arterial hypertension, suppressed plasma renin activity, and increased adrenal aldosterone secretion. The prevalence of PA is between 1.4 and 23%, depending on the population investigated and on the selected criteria for its diagnosis (higher prevalence in resistant hypertensives). According to a large prospective study in newly diagnosed hypertensive patients, the prevalence is about 11% and the most common causes for primary aldosteronism are aldosterone-producing adenoma (APA; 4.8%) and idiopathic hyperaldosteronism (IHA, 6.4%) or idiopathic adrenal hyperplasia.68 Glucocorticoid remediable aldosteronism is a rare form.
Medical history, clinical findings, and screening work-up in patients with suspected primary aldosteronism. PRA, plasma renin activity; PAC, plasma aldosterone concentration; ARR, aldosterone–renin ratio; 24 h-ABPM, 24 h ambulatory blood-pressure monitoring; LV, left ventricular; K+, potassium; Na+, sodium; Mg2+, magnesium; APA, aldosterone-producing adenoma; IHA, idiopathic hyperaldosteronism.
Clinical clues are not very specific and only about 40% of patients with PA have hypokalaemia.69 Other clinical features are resistant hypertension, muscle weakness, constipation, and fatigue. Hypokalaemia may be accompanied by metabolic alkalosis, excessive urinary sodium excretion, and hypernatraemia. If PA is suspected the plasma aldosterone-renin ratio (ARR) should be assessed as a first screening step. Since ARR is affected by several factors such as antihypertensive medications (Table 5), patients should be prepared for ARR measurement (Table 6).70 Depending on the method used for assessing ARR, the cut-off values for the diagnosis of PA vary (Table 7). If ARR is increased confirmatory tests with sodium loading or captopril suppression should be performed. There are several methods for sodium loading testing.70 The shortest and easiest one is the measurement of plasma aldosterone before and after i.v. infusion of 2000 mL 0.9% saline over 4 h.71 If the post-infusion plasma aldosterone concentration is <5 ng/dL, PA is very unlikely, if it is >10 ng/dL PA is very likely.72 It is important to note that the saline infusion test is in general a safe and specific test to confirm (or exclude) PA, but that it has no place for discriminating between APA and IHA.71 If a confirmatory test is positive, imaging (CT or MRI) and selective vein sampling to should be performed.
Medications that may affect plasma aldosterone, renin, aldosterone–renin ratio, and metanephrine
| Medications . | Aldosterone . | Renin . | ARR . | Metanephrine . |
|---|---|---|---|---|
| β-Blockers | ↓ | ↓↓ | ↑ | ↑ |
| Clonidine (α2-agonist) | ↓ | ↓↓ | ↑ | ↓ |
| α1-Blockers (doxazosin) | → | → | → | → |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ | →↑ |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ | →↑ |
| ACE-inhibitors | ↓ | ↑↑ | ↓ | → |
| ARBs | ↓ | ↑↑ | ↓ | → |
| Ca2+-blockers | →↓ | ↑ | ↓ | → |
| Aliskiren (direct renin inhibitor) | ↓ | ↓ (PRA); ↑ (DRC) | ↑ (PRA); ↓ (DRC) | ↑ |
| Medications . | Aldosterone . | Renin . | ARR . | Metanephrine . |
|---|---|---|---|---|
| β-Blockers | ↓ | ↓↓ | ↑ | ↑ |
| Clonidine (α2-agonist) | ↓ | ↓↓ | ↑ | ↓ |
| α1-Blockers (doxazosin) | → | → | → | → |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ | →↑ |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ | →↑ |
| ACE-inhibitors | ↓ | ↑↑ | ↓ | → |
| ARBs | ↓ | ↑↑ | ↓ | → |
| Ca2+-blockers | →↓ | ↑ | ↓ | → |
| Aliskiren (direct renin inhibitor) | ↓ | ↓ (PRA); ↑ (DRC) | ↑ (PRA); ↓ (DRC) | ↑ |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ARR, aldosterone–renin ratio; PRA, plasma renin activity; DRC, direct renin concentration.
Medications that may affect plasma aldosterone, renin, aldosterone–renin ratio, and metanephrine
| Medications . | Aldosterone . | Renin . | ARR . | Metanephrine . |
|---|---|---|---|---|
| β-Blockers | ↓ | ↓↓ | ↑ | ↑ |
| Clonidine (α2-agonist) | ↓ | ↓↓ | ↑ | ↓ |
| α1-Blockers (doxazosin) | → | → | → | → |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ | →↑ |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ | →↑ |
| ACE-inhibitors | ↓ | ↑↑ | ↓ | → |
| ARBs | ↓ | ↑↑ | ↓ | → |
| Ca2+-blockers | →↓ | ↑ | ↓ | → |
| Aliskiren (direct renin inhibitor) | ↓ | ↓ (PRA); ↑ (DRC) | ↑ (PRA); ↓ (DRC) | ↑ |
| Medications . | Aldosterone . | Renin . | ARR . | Metanephrine . |
|---|---|---|---|---|
| β-Blockers | ↓ | ↓↓ | ↑ | ↑ |
| Clonidine (α2-agonist) | ↓ | ↓↓ | ↑ | ↓ |
| α1-Blockers (doxazosin) | → | → | → | → |
| K+-wasting diuretics | →↑ | ↑↑ | ↓ | →↑ |
| K+-sparing diuretics | ↑ | ↑↑ | ↓ | →↑ |
| ACE-inhibitors | ↓ | ↑↑ | ↓ | → |
| ARBs | ↓ | ↑↑ | ↓ | → |
| Ca2+-blockers | →↓ | ↑ | ↓ | → |
| Aliskiren (direct renin inhibitor) | ↓ | ↓ (PRA); ↑ (DRC) | ↑ (PRA); ↓ (DRC) | ↑ |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ARR, aldosterone–renin ratio; PRA, plasma renin activity; DRC, direct renin concentration.
Preparation for the measurement of ARRa
| Correct hypokalaemia if present and liberalize sodium intake |
| Change antihypertensive medication to drugs with minimal effects on ARR: |
| Verapamil slow-release (90–120 mg twice daily) |
| Hydralazine (10–12.5 mg twice daily) |
| Doxazosin (2–8 mg once daily) |
| Blood sampling: |
| Collect blood in the mid-morning, after the patient has been out of bed for at least 2 h and sitting 5–15 min |
| Avoid stasis and haemolysis |
| Maintain blood at room temperature |
| Correct hypokalaemia if present and liberalize sodium intake |
| Change antihypertensive medication to drugs with minimal effects on ARR: |
| Verapamil slow-release (90–120 mg twice daily) |
| Hydralazine (10–12.5 mg twice daily) |
| Doxazosin (2–8 mg once daily) |
| Blood sampling: |
| Collect blood in the mid-morning, after the patient has been out of bed for at least 2 h and sitting 5–15 min |
| Avoid stasis and haemolysis |
| Maintain blood at room temperature |
ARR, aldosterone:renin ratio.
aModified after reference (56).
Preparation for the measurement of ARRa
| Correct hypokalaemia if present and liberalize sodium intake |
| Change antihypertensive medication to drugs with minimal effects on ARR: |
| Verapamil slow-release (90–120 mg twice daily) |
| Hydralazine (10–12.5 mg twice daily) |
| Doxazosin (2–8 mg once daily) |
| Blood sampling: |
| Collect blood in the mid-morning, after the patient has been out of bed for at least 2 h and sitting 5–15 min |
| Avoid stasis and haemolysis |
| Maintain blood at room temperature |
| Correct hypokalaemia if present and liberalize sodium intake |
| Change antihypertensive medication to drugs with minimal effects on ARR: |
| Verapamil slow-release (90–120 mg twice daily) |
| Hydralazine (10–12.5 mg twice daily) |
| Doxazosin (2–8 mg once daily) |
| Blood sampling: |
| Collect blood in the mid-morning, after the patient has been out of bed for at least 2 h and sitting 5–15 min |
| Avoid stasis and haemolysis |
| Maintain blood at room temperature |
ARR, aldosterone:renin ratio.
aModified after reference (56).
Aldosterone–renin ratio cut-off values for the diagnosis of primary aldosteronism
| Plasma aldosterone (PAC) . | Plasma renin . | |||
|---|---|---|---|---|
| Activity (PRA) . | Direct concentration (DRC) . | |||
| ng/mL h . | pmol/L min . | mU/L . | ng/L . | |
| ng/dL | >27 | >2.1 | >3.3 | >5.4 |
| pmol/L | >750 | >59 | >90 | >150 |
| Plasma aldosterone (PAC) . | Plasma renin . | |||
|---|---|---|---|---|
| Activity (PRA) . | Direct concentration (DRC) . | |||
| ng/mL h . | pmol/L min . | mU/L . | ng/L . | |
| ng/dL | >27 | >2.1 | >3.3 | >5.4 |
| pmol/L | >750 | >59 | >90 | >150 |
ARR, aldosterone-renin ratio (the most commonly used cut-off values for the diagnosis of primary aldosteronism are showed in bold); PAC, plasma aldosterone concentration; PRA, plasma renin activity; DRC, direct renin concentration.
Conversion factor: aldosterone 1 ng/dL → 27.7 pmol/L. Conversion factor PRA 1 ng/mL h → 1 pmol/L min divided by 12.8. Conversion factor: PRA 1 ng/mL h → DRC 1 mU/L divided by 8.2. Conversion factor: PRA 1 ng/mL h → 1DRC ng/L divided by 5.2.
Aldosterone–renin ratio cut-off values for the diagnosis of primary aldosteronism
| Plasma aldosterone (PAC) . | Plasma renin . | |||
|---|---|---|---|---|
| Activity (PRA) . | Direct concentration (DRC) . | |||
| ng/mL h . | pmol/L min . | mU/L . | ng/L . | |
| ng/dL | >27 | >2.1 | >3.3 | >5.4 |
| pmol/L | >750 | >59 | >90 | >150 |
| Plasma aldosterone (PAC) . | Plasma renin . | |||
|---|---|---|---|---|
| Activity (PRA) . | Direct concentration (DRC) . | |||
| ng/mL h . | pmol/L min . | mU/L . | ng/L . | |
| ng/dL | >27 | >2.1 | >3.3 | >5.4 |
| pmol/L | >750 | >59 | >90 | >150 |
ARR, aldosterone-renin ratio (the most commonly used cut-off values for the diagnosis of primary aldosteronism are showed in bold); PAC, plasma aldosterone concentration; PRA, plasma renin activity; DRC, direct renin concentration.
Conversion factor: aldosterone 1 ng/dL → 27.7 pmol/L. Conversion factor PRA 1 ng/mL h → 1 pmol/L min divided by 12.8. Conversion factor: PRA 1 ng/mL h → DRC 1 mU/L divided by 8.2. Conversion factor: PRA 1 ng/mL h → 1DRC ng/L divided by 5.2.
Relying on imaging only may lead to inappropriate treatment of patients with primary aldosteronism. CT/MRI misdiagnosed the cause of primary aldosteronism in 37.8% of patients when adrenal vein sampling was used as the standard test for diagnosing laterality of aldosterone secretion.73
Uncommon causes of secondary hypertension
Cushing's syndrome
Cushing's syndrome is a rare syndrome affecting <0.1% of the general population.74 Patients with this syndrome display a typical body habitus with obesity, facial plethora, buffalo hump, hirsutism, and purple striae.75 Hypertension is very common affecting about 80% of patients with Cushing. A 24 h urinary cortisol excretion >55ug/24 h is suggestive for Cushing. Further work up includes 1 mg dexamethasone suppression test at bedtime with measurement of cortisol plasma concentration the next morning (cut-off 1.8 μg/dL).76
Hyper-/hypothyroidism
Both hyper- and hypothyroidism have been associated with arterial hypertension. In hypothyroidism, diastolic BP is particularly elevated since low cardiac output is compensated by peripheral vasoconstriction to maintain adequate tissue perfusion. In contrast, hyperthyroidism is associated with increased cardiac output and predominately systolic BP elevation. The best screening test is thyroid-stimulating hormone plasma concentration.
Phaeochromocytoma
Phaeochromocytoma has a prevalence of about 0.2% in unselected hypertensive patients. Clinical features are due to the paroxysmal increase of plasma catecholamines and are characterized by the ‘five P’77: Screening for phaeochromocytoma should be performed only if one or more of the following criteria are present: resistant hypertension and hyperadrenergic spells (the ‘five P’); family history of phaeochromocytoma; genetic syndrome known to be associated with phaeochromocytoma (MEN 2; von Hippel Lindau, neurofibromatosis), adrenal mass with characteristics consistent with phaeochromocytoma (i.e. large size >4 cm, cystic and haemorrhagic changes).
paroxysmal hypertension;
palpitation;
perspiration;
pallor;
pounding headache.
Two main screening tests are available: 24 h urine catecholamines and metanephrine or plasma fractionated metanephrines. Some antihypertensive drugs may have effects on metanephrine plasma levels (Table 5). If screening is positive, imaging with abdominal/adrenal MRI or CT is indicated. If abdominal imaging is negative, scintigraphic localization with 123I-metaiodobenzylguanidine (MIBG) or additional imaging (i.e. whole body MRI, or others) is indicated.
Coarctation of the aorta
Aortic coarctation is the second most common cause of hypertension in children and young adults.9 It is characterized by constriction of the lumen of the aorta usually near the ligamentum arteriosum. This lesion makes up for about 7% of all cases of congenital heart disease.78 Frequent symptoms are headache, cold feet, and pain in the legs during exercise. Clinical clues are arterial hypertension in the presence of weak femoral pulses. Other typical findings include systolic murmurs in the front and/or back of the chest and notching of the posterior ribs (collateral circulation) on a chest radiograph. Echocardiography is the screening method of choice. Alternatively CT or MRI may also be performed. Early surgical repair or percutaneous balloon angioplasty appears equally effective.79 According to the current guidelines,80 patients with coarctation need regular follow-up at least every 2 years in a specialized centre for adult patients with congenital heart disease. Follow-up should include echocardiography and assessment of BP (preferably 24 h ABPM with the cuff placed on the right upper arm). Imaging intervals of the aorta (preferably with MRI) depend on baseline pathology. Even less common than coarctation of the thoracic aorta is coarctation of the abdominal aorta which can give rise to severe hypertension at an early age.81
Long-term follow-up is recommended for these patients who are at risk for persistent hypertension and other cardiovascular complications.81–83
Conclusion
Secondary hypertension affects only 5–10% of the hypertensive patients. Screening is expensive and time-consuming and should be performed only in patients with high clinical suspicion. Moreover, despite having found and appropriately treated a secondary cause of hypertension, BP rarely ever returns to normal. This indicates either that some patients with secondary hypertension also have concomitant essential hypertension or that vascular remodelling has taken place and progressed over time to the point of no return. Therefore, in patients with a potentially reversible cause of hypertension, early detection and treatment are important to minimize/prevent irreversible changes in the systemic vasculature which may give rise to persistent hypertension with an unfavourable long-term outcome.
Funding
We gratefully acknowledge support from the Swiss National Science Foundation and the Swiss Society of Hypertension.
Conflict of interest: none declared.