-
PDF
- Split View
-
Views
-
Cite
Cite
Monica Gianni, Francesco Dentali, Anna Maria Grandi, Glen Sumner, Rajesh Hiralal, Eva Lonn, Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review, European Heart Journal, Volume 27, Issue 13, July 2006, Pages 1523–1529, https://doi.org/10.1093/eurheartj/ehl032
Close - Share Icon Share
Abstract
Aims To clarify the major features of the apical ballooning syndrome, we performed a systematic review of the existing literature.
Methods and results Review of all relevant case series using the MEDLINE and EMBASE databases resulted in the identification of 14 studies. These studies suggest that the apical ballooning syndrome accounts for ∼2.0% of ST-segment elevation infarcts, with most cases described in post-menopausal women. The most common clinical presentations are chest pain and dyspnoea, reported in 67.8 and 17.8% of the patients, respectively. Cardiogenic shock (4.2% of the patients) and ventricular fibrillation (1.5%) were not infrequent. ST-segment elevation was reported in 81.6% of the patients, T wave abnormalities in 64.3%, and Q waves in 31.8%. Cardiac biomarkers were usually mildly elevated, as reported in 86.2% of the patients. Typically, patients had left ventricular (LV) dysfunction on admission, with mean ejection fraction ranging from 20 to 49%. However, over a period of days to weeks, all patients experienced dramatic improvement in LV function. The onset of symptoms was often preceded by emotional (26.8%) or physical stress (37.8%). Norepinephrine concentration was elevated in 74.3% of the patients. Prognosis was generally excellent, with full recovery in most patients. In-hospital mortality was 1.1%. Only 3.5% of the patients experienced a recurrence.
Conculsion Clinicians should consider this syndrome in the differential diagnosis of patients presenting with chest pain, especially in post-menopausal women with a recent history of emotional or physical stress.
Introduction
Transient left ventricular (LV) apical ballooning syndrome is a cardiac syndrome characterized by transient LV dysfunction, electrocardiographic changes that can mimic acute myocardial infarction (MI), and minimal release of myocardial enzymes in the absence of obstructive coronary artery disease (CAD).1 This syndrome was first described in 1991 in Japan and named takotsubo-like LV dysfunction in reference to the associated LV morphological features.1 Takotsubo is a pot with a round bottom and narrow neck used for trapping octopuses in Japan. More recently, the condition has been called transient LV apical ballooning syndrome or ampulla cardiomyopathy in relation to the balloon-like wall-motion abnormalities involving the LV apex.2 Since 1991, several case reports and small series have described the affected patients in Japan.1–4 More recently, case series have been reported in Caucasian populations in Europe and North America.5
Owing to its clinical and imaging characteristics, this syndrome is frequently misdiagnosed as an acute coronary syndrome (ACS) related to occlusive epicardial CAD. However, coronary angiography is frequently normal or reveals only mild abnormalities, and typically, patients do not have obstructive coronary lesions (>50% luminal stenosis). Moreover, despite the frequently dramatic clinical presentation, almost all patients recover fully and the LV systolic function, heavily compromised at presentation, improves rapidly in a period of days to weeks.
Several pathophysiological mechanisms have been proposed to explain the unusual features of this syndrome, such as multivessel coronary vasospasm, abnormalities in coronary microvascular function, and catecholamine-mediated cardiotoxicity. However, the precise aetiology and pathophysiology of this syndrome remain unknown.
The information concerning the transient LV apical ballooning syndrome is limited and based mainly on the case reports and case series, frequently comprising few patients. Furthermore, most studies are retrospective and only few have followed patients after hospital discharge.
Therefore, to further clarify the prevalence, clinical characteristics, natural history, prognosis, and pathophysiology of the transient LV apical ballooning syndrome, we performed a systematic research of the existing literature on this topic.
Methods
A computer-assisted search was performed using the MEDLINE (from 1966 to August 2005) and EMBASE (from 1980 to August 2005) databases. The following search terms were used with no language restrictions and combined: transient LV apical ballooning syndrome, takotsubo-like LV dysfunction, ampulla cardiomyopathy, and amphora cardiomyopathy. Criteria for the inclusion of publications in this systematic review were set a priori and were: (i) reporting of original data; (ii) inclusion of at least five patients (reports comprising fewer patients were not included to avoid less reliable data); (iii) all patients had transient akinesis or dyskinesis of the LV apex and wall-motion abnormalities involving the mid-ventricular LV segments, with a pattern of regional wall-motion abnormalities extending beyond the distribution of a single epicardial coronary artery, as diagnosed by echocardiography or left ventriculography; (iv) all patients underwent coronary angiography, which revealed no obstructive coronary disease (normal coronary arteries or luminal stenosis <50%) and no acute plaque rupture; and (v) the study provided data of at least one of the following: prevalence, clinical presentation, natural history, long-term prognosis, and underlying pathophysiological mechanisms of the apical ballooning syndrome. Publications that included patients with other conditions who might present with clinical and imaging abnormalities similar to the transient LV apical ballooning syndrome (intracranial or subarachnoid bleeding, pheochromocytoma, myocarditis, or hypertrophic cardiomyopathy) were excluded. The list of articles generated by the search was manually reviewed by two investigators (M.G. and F.D.). Publications with titles or abstracts suspected to meet the eligibility criteria for this systematic review were selected for detailed review. Additional publications meeting these inclusion criteria were selected for review from the authors' libraries and from review of the reference lists of those articles selected for detailed review. When multiple publications describing the same case series were published, we used the latest publication and supplemented, if necessary, with data from earlier publications. For each study selected, two reviewers (M.G. and F.D.) extracted data independently on prevalence, clinical presentation, in-hospital complications, long-term prognosis, and pathophysiological mechanisms. Disagreements were resolved by consensus or by a third, independent reviewer (G.S.). A formal meta-analysis was not appropriate because of the heterogeneity of the methodology and outcome assessments among the studies. Therefore, a narrative synthesis of the collected data was undertaken. Whenever possible, data are presented as mean values, confidence intervals (CIs), percentages, and ranges of variation.
Results
Studies' characteristics
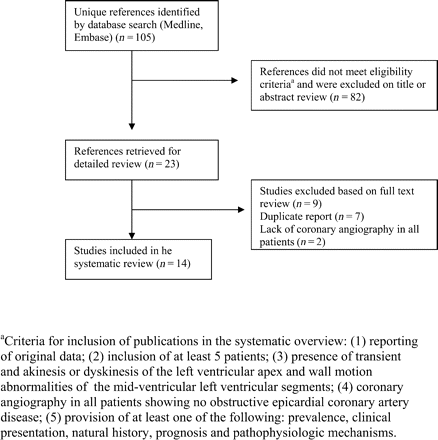
We identified 105 published studies. No unpublished studies meeting our eligibility criteria were located. Eighty-two citations were excluded after examining titles and abstracts, leaving 23 studies for detailed evaluation.1–23 Seven publications were duplicate reports on the same patients1,3,4,10,14–16 and two reports were excluded because they lacked coronary angiography in all patients.17,23 The remaining 14 studies were included in our systematic review2,5–9,11–13,18–22 (Figure 1).
Flow diagram for the selection of studies included in the systematic review.
The main characteristics of the studies included in this review are summarized in Table 1. All publications were written in English. There are nine reports from Japan,2,8,11,13,18–22 three from the USA,6,7,12 one from Belgium,5 and one from Spain.9 Seven studies were prospective2,5–7,9,11,21 and seven were retrospective.8,12,15,18–20,22 All prospective studies evaluated consecutive patients. The studies ranged from nine to 88 patients. A total of 286 patients are included in our review. Wittstein et al.6 included one patient with a coronary luminal narrowing of 70% in the proximal left anterior descending coronary artery. In all other studies, patients with any epicardial coronary artery luminal stenosis of >50% were excluded. Possible pathophysiological mechanisms were evaluated in eight studies.5,6,9,11–13,19,21
Patients' demographics, presenting symptoms, history, electrocardiographic abnormalities, and cardiac biomarkers
| Reference . | No. of patients (women) . | Mean age (range) . | Chest paina . | Dyspnoeaa . | Preceding emotional stressora . | Preceding physiological stressora . | ST-segment elevationa . | ST-segment elevation in pre-cordial leadsa . | Pathological Q wavesa . | Inverted T wavesa . | Elevated CK-MBa . | Elevated troponin I or Ta . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 19 (18) | 58 (27–87) | 18 (95) | NR | 19 (100) | NR | 2 (10.5) | NR | 7 (38) | 18 (95) | NR | 17 (89.5) |
| Sharkey et al.7 | 22 (20) | 65 (32–89) | 20 (91) | 1 (4.5) | 19 (86) | 3 (14) | 13 (59) | 13 (59) | 10 (45) | 15 (68) | NR | 19 (86) |
| Inoue et al.8 | 18 (11) | 76 (60–86) | 11 (61) | NR | 2 (11) | 7 (39) | NR | NR | NR | NR | NR | NR |
| Ibanez et al.9 | 11 (9) | 72 (41–84) | 11 (100) | NR | 3 (27) | 3 (27) | 11 (100) | NR | NR | 9 (82) | NR | 11 (100) |
| Akashi et al.11 | 10 (9) | 73 (57–85) | 2 (20) | 2 (20) | 1 (10) | 5 (50) | 9 (90) | 7 (100) | 10 (100) | 10 (100) | 0 | NR |
| Bybee et al.12 | 16 (11) | 71 (52–88) | 11 (69) | 4 (25) | 6 (38) | 7 (44) | 13 (81) | 13 (81) | 5 (31) | 13 (81) | NR | 16 (100) |
| Kurisu et al.13 | 30 (28) | 70 (55–83) | 19 (63) | 6 (20) | 5 (17) | 5 (17) | 30 (100) | 29 (97) | NR | 13 (43) | NR | NR |
| Matsuoka et al.18 | 10 (9) | 68 (59–83) | 6 (60) | 3 (30) | 3 (30) | 7 (70) | 10 (100) | 10 (100) | NR | NR | NR | NR |
| Desmet et al.5 | 13 (12) | 62 (45–81) | 8 (62) | 4 (31) | 3 (23) | 6 (46) | 8 (62) | 6 (46) | 4 (31) | 12 (92) | 13 (100) | 12/12 (100) |
| Ito et al.19 | 10 (7) | 63 (56–83) | 8 (80) | 4 (40) | 3 (30) | 4 (40) | 10 (100) | 10 (100) | NR | 10 (100) | 4 (40) | NR |
| Ogura et al.20 | 13 (9) | 75±10b | NR | NR | NR | NR | NR | NR | 2 (15) | 4 (31) | NR | NR |
| Abe et al.21 | 17 (14) | 74 (54–91) | 9 (53) | 5 (29) | 3 (17.6) | 6 (35.3) | 14 (82.4) | NR | 1 (6) | 17 (100) | NR | NR |
| Tsuchihashi et al.22 | 88 (76) | 67 (10–88) | 59 (67) | 6 (7) | 18 (20) | 40 (45) | 79 (90) | 75 (85) | 24 (27) | 39 (44) | NR | 31/43 (72) |
| Kawai et al.2 | 9 (9) | 66 (53–82) | 3 (33) | 5 (53) | 2 (22) | 3 (33) | 9 (100) | 9 (100) | NR | NR | NR | NR |
| Reference . | No. of patients (women) . | Mean age (range) . | Chest paina . | Dyspnoeaa . | Preceding emotional stressora . | Preceding physiological stressora . | ST-segment elevationa . | ST-segment elevation in pre-cordial leadsa . | Pathological Q wavesa . | Inverted T wavesa . | Elevated CK-MBa . | Elevated troponin I or Ta . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 19 (18) | 58 (27–87) | 18 (95) | NR | 19 (100) | NR | 2 (10.5) | NR | 7 (38) | 18 (95) | NR | 17 (89.5) |
| Sharkey et al.7 | 22 (20) | 65 (32–89) | 20 (91) | 1 (4.5) | 19 (86) | 3 (14) | 13 (59) | 13 (59) | 10 (45) | 15 (68) | NR | 19 (86) |
| Inoue et al.8 | 18 (11) | 76 (60–86) | 11 (61) | NR | 2 (11) | 7 (39) | NR | NR | NR | NR | NR | NR |
| Ibanez et al.9 | 11 (9) | 72 (41–84) | 11 (100) | NR | 3 (27) | 3 (27) | 11 (100) | NR | NR | 9 (82) | NR | 11 (100) |
| Akashi et al.11 | 10 (9) | 73 (57–85) | 2 (20) | 2 (20) | 1 (10) | 5 (50) | 9 (90) | 7 (100) | 10 (100) | 10 (100) | 0 | NR |
| Bybee et al.12 | 16 (11) | 71 (52–88) | 11 (69) | 4 (25) | 6 (38) | 7 (44) | 13 (81) | 13 (81) | 5 (31) | 13 (81) | NR | 16 (100) |
| Kurisu et al.13 | 30 (28) | 70 (55–83) | 19 (63) | 6 (20) | 5 (17) | 5 (17) | 30 (100) | 29 (97) | NR | 13 (43) | NR | NR |
| Matsuoka et al.18 | 10 (9) | 68 (59–83) | 6 (60) | 3 (30) | 3 (30) | 7 (70) | 10 (100) | 10 (100) | NR | NR | NR | NR |
| Desmet et al.5 | 13 (12) | 62 (45–81) | 8 (62) | 4 (31) | 3 (23) | 6 (46) | 8 (62) | 6 (46) | 4 (31) | 12 (92) | 13 (100) | 12/12 (100) |
| Ito et al.19 | 10 (7) | 63 (56–83) | 8 (80) | 4 (40) | 3 (30) | 4 (40) | 10 (100) | 10 (100) | NR | 10 (100) | 4 (40) | NR |
| Ogura et al.20 | 13 (9) | 75±10b | NR | NR | NR | NR | NR | NR | 2 (15) | 4 (31) | NR | NR |
| Abe et al.21 | 17 (14) | 74 (54–91) | 9 (53) | 5 (29) | 3 (17.6) | 6 (35.3) | 14 (82.4) | NR | 1 (6) | 17 (100) | NR | NR |
| Tsuchihashi et al.22 | 88 (76) | 67 (10–88) | 59 (67) | 6 (7) | 18 (20) | 40 (45) | 79 (90) | 75 (85) | 24 (27) | 39 (44) | NR | 31/43 (72) |
| Kawai et al.2 | 9 (9) | 66 (53–82) | 3 (33) | 5 (53) | 2 (22) | 3 (33) | 9 (100) | 9 (100) | NR | NR | NR | NR |
NR, not reported.
aValues expressed as number and percentages.
bValues expressed as mean±SD.
Patients' demographics, presenting symptoms, history, electrocardiographic abnormalities, and cardiac biomarkers
| Reference . | No. of patients (women) . | Mean age (range) . | Chest paina . | Dyspnoeaa . | Preceding emotional stressora . | Preceding physiological stressora . | ST-segment elevationa . | ST-segment elevation in pre-cordial leadsa . | Pathological Q wavesa . | Inverted T wavesa . | Elevated CK-MBa . | Elevated troponin I or Ta . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 19 (18) | 58 (27–87) | 18 (95) | NR | 19 (100) | NR | 2 (10.5) | NR | 7 (38) | 18 (95) | NR | 17 (89.5) |
| Sharkey et al.7 | 22 (20) | 65 (32–89) | 20 (91) | 1 (4.5) | 19 (86) | 3 (14) | 13 (59) | 13 (59) | 10 (45) | 15 (68) | NR | 19 (86) |
| Inoue et al.8 | 18 (11) | 76 (60–86) | 11 (61) | NR | 2 (11) | 7 (39) | NR | NR | NR | NR | NR | NR |
| Ibanez et al.9 | 11 (9) | 72 (41–84) | 11 (100) | NR | 3 (27) | 3 (27) | 11 (100) | NR | NR | 9 (82) | NR | 11 (100) |
| Akashi et al.11 | 10 (9) | 73 (57–85) | 2 (20) | 2 (20) | 1 (10) | 5 (50) | 9 (90) | 7 (100) | 10 (100) | 10 (100) | 0 | NR |
| Bybee et al.12 | 16 (11) | 71 (52–88) | 11 (69) | 4 (25) | 6 (38) | 7 (44) | 13 (81) | 13 (81) | 5 (31) | 13 (81) | NR | 16 (100) |
| Kurisu et al.13 | 30 (28) | 70 (55–83) | 19 (63) | 6 (20) | 5 (17) | 5 (17) | 30 (100) | 29 (97) | NR | 13 (43) | NR | NR |
| Matsuoka et al.18 | 10 (9) | 68 (59–83) | 6 (60) | 3 (30) | 3 (30) | 7 (70) | 10 (100) | 10 (100) | NR | NR | NR | NR |
| Desmet et al.5 | 13 (12) | 62 (45–81) | 8 (62) | 4 (31) | 3 (23) | 6 (46) | 8 (62) | 6 (46) | 4 (31) | 12 (92) | 13 (100) | 12/12 (100) |
| Ito et al.19 | 10 (7) | 63 (56–83) | 8 (80) | 4 (40) | 3 (30) | 4 (40) | 10 (100) | 10 (100) | NR | 10 (100) | 4 (40) | NR |
| Ogura et al.20 | 13 (9) | 75±10b | NR | NR | NR | NR | NR | NR | 2 (15) | 4 (31) | NR | NR |
| Abe et al.21 | 17 (14) | 74 (54–91) | 9 (53) | 5 (29) | 3 (17.6) | 6 (35.3) | 14 (82.4) | NR | 1 (6) | 17 (100) | NR | NR |
| Tsuchihashi et al.22 | 88 (76) | 67 (10–88) | 59 (67) | 6 (7) | 18 (20) | 40 (45) | 79 (90) | 75 (85) | 24 (27) | 39 (44) | NR | 31/43 (72) |
| Kawai et al.2 | 9 (9) | 66 (53–82) | 3 (33) | 5 (53) | 2 (22) | 3 (33) | 9 (100) | 9 (100) | NR | NR | NR | NR |
| Reference . | No. of patients (women) . | Mean age (range) . | Chest paina . | Dyspnoeaa . | Preceding emotional stressora . | Preceding physiological stressora . | ST-segment elevationa . | ST-segment elevation in pre-cordial leadsa . | Pathological Q wavesa . | Inverted T wavesa . | Elevated CK-MBa . | Elevated troponin I or Ta . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 19 (18) | 58 (27–87) | 18 (95) | NR | 19 (100) | NR | 2 (10.5) | NR | 7 (38) | 18 (95) | NR | 17 (89.5) |
| Sharkey et al.7 | 22 (20) | 65 (32–89) | 20 (91) | 1 (4.5) | 19 (86) | 3 (14) | 13 (59) | 13 (59) | 10 (45) | 15 (68) | NR | 19 (86) |
| Inoue et al.8 | 18 (11) | 76 (60–86) | 11 (61) | NR | 2 (11) | 7 (39) | NR | NR | NR | NR | NR | NR |
| Ibanez et al.9 | 11 (9) | 72 (41–84) | 11 (100) | NR | 3 (27) | 3 (27) | 11 (100) | NR | NR | 9 (82) | NR | 11 (100) |
| Akashi et al.11 | 10 (9) | 73 (57–85) | 2 (20) | 2 (20) | 1 (10) | 5 (50) | 9 (90) | 7 (100) | 10 (100) | 10 (100) | 0 | NR |
| Bybee et al.12 | 16 (11) | 71 (52–88) | 11 (69) | 4 (25) | 6 (38) | 7 (44) | 13 (81) | 13 (81) | 5 (31) | 13 (81) | NR | 16 (100) |
| Kurisu et al.13 | 30 (28) | 70 (55–83) | 19 (63) | 6 (20) | 5 (17) | 5 (17) | 30 (100) | 29 (97) | NR | 13 (43) | NR | NR |
| Matsuoka et al.18 | 10 (9) | 68 (59–83) | 6 (60) | 3 (30) | 3 (30) | 7 (70) | 10 (100) | 10 (100) | NR | NR | NR | NR |
| Desmet et al.5 | 13 (12) | 62 (45–81) | 8 (62) | 4 (31) | 3 (23) | 6 (46) | 8 (62) | 6 (46) | 4 (31) | 12 (92) | 13 (100) | 12/12 (100) |
| Ito et al.19 | 10 (7) | 63 (56–83) | 8 (80) | 4 (40) | 3 (30) | 4 (40) | 10 (100) | 10 (100) | NR | 10 (100) | 4 (40) | NR |
| Ogura et al.20 | 13 (9) | 75±10b | NR | NR | NR | NR | NR | NR | 2 (15) | 4 (31) | NR | NR |
| Abe et al.21 | 17 (14) | 74 (54–91) | 9 (53) | 5 (29) | 3 (17.6) | 6 (35.3) | 14 (82.4) | NR | 1 (6) | 17 (100) | NR | NR |
| Tsuchihashi et al.22 | 88 (76) | 67 (10–88) | 59 (67) | 6 (7) | 18 (20) | 40 (45) | 79 (90) | 75 (85) | 24 (27) | 39 (44) | NR | 31/43 (72) |
| Kawai et al.2 | 9 (9) | 66 (53–82) | 3 (33) | 5 (53) | 2 (22) | 3 (33) | 9 (100) | 9 (100) | NR | NR | NR | NR |
NR, not reported.
aValues expressed as number and percentages.
bValues expressed as mean±SD.
Prevalence
The true prevalence of the apical ballooning syndrome remains uncertain. In the last few years, the number of published reports of patients presenting with this syndrome is constantly increasing. Only four series assessed the prevalence of this syndrome among consecutive patients presenting with suspected ACS.11,12,18,19 In a recent study from the USA, Bybee et al.12 reported that the apical ballooning syndrome accounted for ∼2.2% of the ST-segment elevation ACS presenting to the investigators' institution in the years 2002 and 2003. Three series evaluated the prevalence of this syndrome in Japan.11,18,19 Among patients presenting with suspected ACS, Ito et al.19 reported that the apical ballooning syndrome accounted for 1.7% of cases and Matsuoka et al.18 for 2.2%. Akashi et al.11 diagnosed apical ballooning syndrome in 2.0% of patients with sudden onset of heart failure and abnormal Q waves or ST-T changes suggestive of acute MI on admission.
Patients' demographics and presenting symptoms
All the 14 studies included in our systematic review showed a marked gender discrepancy, with much more common involvement of women. Two hundred and fifty-four of 286 patients (88.8%) were women (95% CI: 84.6–92.0%) (Table 1). Patients' age ranged from 10 to 89 years and mean age in the studies reviewed ranged from 58 to 77 years. Twelve studies provided data about the age distribution of the population. Among these, only five of 185 evaluated patients (2.7%, 95% CI: 1.2–6.2%) were younger than 50 years. Eleven of the 14 studies evaluated risk factors for atherosclerotic coronary heart disease. Collectively these studies reported a history of hypertension in 43% of patients (108/247), diabetes in 11.0% (26/237), dyslipidaemia in 25.45% (53/217), and current or past smoking in 23% (23/100).
The most common presenting clinical symptoms were chest pain and dyspnoea. However, the presence or the absence of these symptoms was not reported for all patients in all the studies reviewed. Chest pain was reported to be a cardinal presenting symptom in 185 of 273 patients (67.8%, 95% CI: 62.0–73.0%; range: 20–94.7%) and dyspnoea in 40 of 225 patients (17.8%, 95% CI: 13.3–23.3%; range: 4.5–55.5%). However, more serious clinical presentations such as cardiogenic shock and ventricular fibrillation are frequent, 4.2% (95% CI: 2.4–7.4%) and 1.5% (95% CI: 0.65–3.9%), respectively.
The onset of the transient LV apical ballooning syndrome is often preceded by emotional or physical stress. An emotional stressor, such as unexpected death of a relative or friend, domestic abuse, confrontational arguments, a catastrophic medical diagnosis, devastating business, or gambling losses, was identified in 68 of 254 patients (26.8%, 95% CI: 21.7–32.5%; range: 10–100%) and a physical stressor, such as exhausting work, asthma attack, gastric endoscopy, and exacerbated systemic disorders in 96 of 254 patients (37.8%, 95% CI: 32.1–43.9%; range: 14–70%), in whom the presence or the absence of a stressor was reported. However, in 87 of 212 patients (34.3%, 95% CI: 28.7–40.3%; range: 0–100%), there was no preceding emotional or physical stressful event identified.
Only eight studies specified treatment in the acute phase and very few reported on ongoing medical treatment. Only one patient received thrombolysis and none received percutaneous coronary intervention; 23 of 212 patients were treated with intra-aortic balloon counterpulsation.
Electrocardiographic features and cardiac biomarkers
The most common abnormalities were ST-segment elevation and T wave inversion, usually observed during the acute and subacute phases. ST-elevation was detected in 208 of 255 evaluated patients (81.6%, 95% CI: 76.4–85.9%; range: 10.5–100%), usually involving the pre-cordial leads, 172 of 205 patients (83.9%, 95% CI: 78.3–88.3%; range: 46.2–100%). T wave abnormalities were seen in 160 of 249 patients (64.3%, 95% CI: 58.1–70.0%; range: 31–100%) and Q waves were present in 63 of 198 evaluated patients (31.8%, 95% CI: 25.7–38.3%; range: 6–100%).
Six studies measured serum levels of troponin I or T5–7,9,12,22 and three CK-MB fraction levels.5,14,19 Troponin was positive in 106 of 123 patients (86.2%, 95% CI: 79.0–91.2%; range: 72–100%) and CK-MB levels were elevated in 17 of 23 (73.9%, 95% CI: 53.5–87.5%; range: 40–100%). However, cardiac biomarker levels were usually only slightly elevated.
Cardiac catheterization and echocardiography
Four studies specified that coronary angiography was performed in all patients and that no significant obstructive CAD was identified, but did not provide further details. In the remaining 10 studies, most patients (108 of 134) had normal coronary arteries (80.6%, 95% CI: 73.1–86.4%; range: 25–100%) and the others had mild luminal stenosis (<50% luminal stenosis) (Table 2). A transient, dynamic intraventricular pressure gradient was found in 21 of 133 evaluated patients (15.8%, range: 12.5–23%). Nine studies6,7,9,11–13,18,19,22 provided data on mean LV ejection fraction (EF) at presentation and 10 studies2,6,7,9,11–13,18,19,22 at follow-up (range: 7–53 days). LV function was generally assessed by ventriculography, echocardiography, or both. Usually, patients had marked LV dysfunction on admission, mean EF ranging from 20 to 49%. However, over a period of days to weeks, all patients experienced a dramatic improvement in their LV function, with mean EF at follow-up ranging from 60 to 76%. During the acute phase, all patients had moderate-to-severe mid-ventricular dysfunction and apical akinesis or diskinesis with the basal function preserved or hyperkinetic (Figure 1). Mid-ventricle and apical wall-motion abnormalities completely resolved in all surviving patients.
LV function on echocardiography or ventriculography at presentation and in follow-up, coronary angiography, haemodynamic findings, and prognosis
| Reference . | Initial LVEFa (%) . | Follow-up LVEFa (%) . | Angiographically normal coronary arteriesb,c . | Transient dynamic intraventricular gradienta . | Heart failure or pulmonary oedemab . | In-hospital mortalityb . | Patients with documented full recoveryb . | Time to recovery in days (range)a . | Documented recurrenceb . |
|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 20 (15–30) | 60 (55–65) | 18 (95) | NR | 3 (16) | 0 | 19/19 (100) | NR | 0 |
| Sharkey et al.7 | 29±9 | 63±6 | NRd | 5 (23) | 0 | 0 | 22/22 (100) | 24±29b | 2 (9) |
| Inoue et al.8 | NR | NR | NRd | NR | 1 (5.6) | 1 (5.6) | NR | NR | NR |
| Ibanez et al.9 | 37 (20–50) | 64 (58–75) | 5/5 (100) | NR | NR | 0 | 11/11 (100) | 13 (3–31) | NR |
| Akashi et al.11 | 42.2 (31–61) | 64.4 (50–75) | 10 (100) | NR | 0 | 0 | 10/10 (100) | 8–29 | 0 |
| Bybee et al.12 | 39.5 (32.8–48) | 60 (50–68) | 4 (25) | 2 (12.5) | 7 (44) | 0 | 13/13 (100) | 8 | 1 (6) |
| Kurisu et al.13 | 49±12a | 69±12a | 25 (83) | NR | 1 (3.3) | 0 | 28/28 (100) | 11.3±4.3b | NR |
| Matsuoka et al.18 | 48±7a | 72±5a | 10 (100) | NR | NR | 0 | 10/10 (100) | 16.1 (7–27) | NR |
| Desmet et al.5 | NR | NR | 5 (38) | 2 (15) | 6 (50) | 1 (7.7) | 12/13 (92) | 27 (9–46) | 2 (15) |
| Ito et al.19 | 43.8±4.3a | 72.3±4.5a | NRd | NR | NR | 0 | 10/10 (100) | NR | NR |
| Ogura et al.20 | NR | NR | 13 (100) | NR | NR | 0 | 13/13 (100) | NR | NR |
| Abe et al.21 | NR | NR | 9/9 (100) | NR | NR | 0 | NR | 9–53 | 0 |
| Tsuchihashi et al.22 | 41 (10–62) | 64 (44–88) | NRd | 12/72 (18) | 20 (23) | 1 (1) | 85/88 (97) | NR | 2/72 (2.7) |
| Kawai et al.2 | NR | 76 (61–86) | 9 (100) | NR | NR | 0 | 9/9 (100) | 8–35 | NR |
| Reference . | Initial LVEFa (%) . | Follow-up LVEFa (%) . | Angiographically normal coronary arteriesb,c . | Transient dynamic intraventricular gradienta . | Heart failure or pulmonary oedemab . | In-hospital mortalityb . | Patients with documented full recoveryb . | Time to recovery in days (range)a . | Documented recurrenceb . |
|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 20 (15–30) | 60 (55–65) | 18 (95) | NR | 3 (16) | 0 | 19/19 (100) | NR | 0 |
| Sharkey et al.7 | 29±9 | 63±6 | NRd | 5 (23) | 0 | 0 | 22/22 (100) | 24±29b | 2 (9) |
| Inoue et al.8 | NR | NR | NRd | NR | 1 (5.6) | 1 (5.6) | NR | NR | NR |
| Ibanez et al.9 | 37 (20–50) | 64 (58–75) | 5/5 (100) | NR | NR | 0 | 11/11 (100) | 13 (3–31) | NR |
| Akashi et al.11 | 42.2 (31–61) | 64.4 (50–75) | 10 (100) | NR | 0 | 0 | 10/10 (100) | 8–29 | 0 |
| Bybee et al.12 | 39.5 (32.8–48) | 60 (50–68) | 4 (25) | 2 (12.5) | 7 (44) | 0 | 13/13 (100) | 8 | 1 (6) |
| Kurisu et al.13 | 49±12a | 69±12a | 25 (83) | NR | 1 (3.3) | 0 | 28/28 (100) | 11.3±4.3b | NR |
| Matsuoka et al.18 | 48±7a | 72±5a | 10 (100) | NR | NR | 0 | 10/10 (100) | 16.1 (7–27) | NR |
| Desmet et al.5 | NR | NR | 5 (38) | 2 (15) | 6 (50) | 1 (7.7) | 12/13 (92) | 27 (9–46) | 2 (15) |
| Ito et al.19 | 43.8±4.3a | 72.3±4.5a | NRd | NR | NR | 0 | 10/10 (100) | NR | NR |
| Ogura et al.20 | NR | NR | 13 (100) | NR | NR | 0 | 13/13 (100) | NR | NR |
| Abe et al.21 | NR | NR | 9/9 (100) | NR | NR | 0 | NR | 9–53 | 0 |
| Tsuchihashi et al.22 | 41 (10–62) | 64 (44–88) | NRd | 12/72 (18) | 20 (23) | 1 (1) | 85/88 (97) | NR | 2/72 (2.7) |
| Kawai et al.2 | NR | 76 (61–86) | 9 (100) | NR | NR | 0 | 9/9 (100) | 8–35 | NR |
NR, not reported.
aValues expressed as mean±SD.
bValues expressed as number and percentages.
cAll other study patients had mild (<50% luminal stenosis) coronary disease.
dStudy reported that all patients underwent coronary angiography and that there were no significant obstructive coronary lesions, but no further details are provided.
LV function on echocardiography or ventriculography at presentation and in follow-up, coronary angiography, haemodynamic findings, and prognosis
| Reference . | Initial LVEFa (%) . | Follow-up LVEFa (%) . | Angiographically normal coronary arteriesb,c . | Transient dynamic intraventricular gradienta . | Heart failure or pulmonary oedemab . | In-hospital mortalityb . | Patients with documented full recoveryb . | Time to recovery in days (range)a . | Documented recurrenceb . |
|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 20 (15–30) | 60 (55–65) | 18 (95) | NR | 3 (16) | 0 | 19/19 (100) | NR | 0 |
| Sharkey et al.7 | 29±9 | 63±6 | NRd | 5 (23) | 0 | 0 | 22/22 (100) | 24±29b | 2 (9) |
| Inoue et al.8 | NR | NR | NRd | NR | 1 (5.6) | 1 (5.6) | NR | NR | NR |
| Ibanez et al.9 | 37 (20–50) | 64 (58–75) | 5/5 (100) | NR | NR | 0 | 11/11 (100) | 13 (3–31) | NR |
| Akashi et al.11 | 42.2 (31–61) | 64.4 (50–75) | 10 (100) | NR | 0 | 0 | 10/10 (100) | 8–29 | 0 |
| Bybee et al.12 | 39.5 (32.8–48) | 60 (50–68) | 4 (25) | 2 (12.5) | 7 (44) | 0 | 13/13 (100) | 8 | 1 (6) |
| Kurisu et al.13 | 49±12a | 69±12a | 25 (83) | NR | 1 (3.3) | 0 | 28/28 (100) | 11.3±4.3b | NR |
| Matsuoka et al.18 | 48±7a | 72±5a | 10 (100) | NR | NR | 0 | 10/10 (100) | 16.1 (7–27) | NR |
| Desmet et al.5 | NR | NR | 5 (38) | 2 (15) | 6 (50) | 1 (7.7) | 12/13 (92) | 27 (9–46) | 2 (15) |
| Ito et al.19 | 43.8±4.3a | 72.3±4.5a | NRd | NR | NR | 0 | 10/10 (100) | NR | NR |
| Ogura et al.20 | NR | NR | 13 (100) | NR | NR | 0 | 13/13 (100) | NR | NR |
| Abe et al.21 | NR | NR | 9/9 (100) | NR | NR | 0 | NR | 9–53 | 0 |
| Tsuchihashi et al.22 | 41 (10–62) | 64 (44–88) | NRd | 12/72 (18) | 20 (23) | 1 (1) | 85/88 (97) | NR | 2/72 (2.7) |
| Kawai et al.2 | NR | 76 (61–86) | 9 (100) | NR | NR | 0 | 9/9 (100) | 8–35 | NR |
| Reference . | Initial LVEFa (%) . | Follow-up LVEFa (%) . | Angiographically normal coronary arteriesb,c . | Transient dynamic intraventricular gradienta . | Heart failure or pulmonary oedemab . | In-hospital mortalityb . | Patients with documented full recoveryb . | Time to recovery in days (range)a . | Documented recurrenceb . |
|---|---|---|---|---|---|---|---|---|---|
| Wittstein et al.6 | 20 (15–30) | 60 (55–65) | 18 (95) | NR | 3 (16) | 0 | 19/19 (100) | NR | 0 |
| Sharkey et al.7 | 29±9 | 63±6 | NRd | 5 (23) | 0 | 0 | 22/22 (100) | 24±29b | 2 (9) |
| Inoue et al.8 | NR | NR | NRd | NR | 1 (5.6) | 1 (5.6) | NR | NR | NR |
| Ibanez et al.9 | 37 (20–50) | 64 (58–75) | 5/5 (100) | NR | NR | 0 | 11/11 (100) | 13 (3–31) | NR |
| Akashi et al.11 | 42.2 (31–61) | 64.4 (50–75) | 10 (100) | NR | 0 | 0 | 10/10 (100) | 8–29 | 0 |
| Bybee et al.12 | 39.5 (32.8–48) | 60 (50–68) | 4 (25) | 2 (12.5) | 7 (44) | 0 | 13/13 (100) | 8 | 1 (6) |
| Kurisu et al.13 | 49±12a | 69±12a | 25 (83) | NR | 1 (3.3) | 0 | 28/28 (100) | 11.3±4.3b | NR |
| Matsuoka et al.18 | 48±7a | 72±5a | 10 (100) | NR | NR | 0 | 10/10 (100) | 16.1 (7–27) | NR |
| Desmet et al.5 | NR | NR | 5 (38) | 2 (15) | 6 (50) | 1 (7.7) | 12/13 (92) | 27 (9–46) | 2 (15) |
| Ito et al.19 | 43.8±4.3a | 72.3±4.5a | NRd | NR | NR | 0 | 10/10 (100) | NR | NR |
| Ogura et al.20 | NR | NR | 13 (100) | NR | NR | 0 | 13/13 (100) | NR | NR |
| Abe et al.21 | NR | NR | 9/9 (100) | NR | NR | 0 | NR | 9–53 | 0 |
| Tsuchihashi et al.22 | 41 (10–62) | 64 (44–88) | NRd | 12/72 (18) | 20 (23) | 1 (1) | 85/88 (97) | NR | 2/72 (2.7) |
| Kawai et al.2 | NR | 76 (61–86) | 9 (100) | NR | NR | 0 | 9/9 (100) | 8–35 | NR |
NR, not reported.
aValues expressed as mean±SD.
bValues expressed as number and percentages.
cAll other study patients had mild (<50% luminal stenosis) coronary disease.
dStudy reported that all patients underwent coronary angiography and that there were no significant obstructive coronary lesions, but no further details are provided.
Prognosis
The prognosis of patients experiencing this syndrome is generally favourable. In-hospital mortality was reported in three of 286 patients (two died of multiple organ failure and one of ovarian cancer) (1.1%, 95% CI: 0.4–3.0%; range: 0–7.7%) (Table 2). Heart failure with or without pulmonary oedema was the most common clinical complication and was reported in 38 of 215 patients (17.7%, 95% CI: 13.2–23.3%). Only six of 169 evaluated patients (3.5%) experienced a recurrence. However, evaluation of the true recurrence rate is limited, as follow-up was not reported in all patients, and in patients assessed during follow-up, timing of the follow-up assessment varied widely, ranging from 8 days to 4 years.
Pathophysiological mechanisms
Many studies have evaluated the presence of either spontaneous or provocable multivessel epicardial spasm during angiography. Only few patients experienced spontaneous multivessel epicardial spasm during coronarography (three of 212 patients, 1.4%; 95% CI: 0.5–4.1%; range: 0–10%). Some investigators used provocative tests, such as infusion of ergonovine or acetylcholine, to evaluate inducible coronary spasm, with conflicting results. Overall, 24 of 84 evaluated patients (28.6%, 95% CI: 20.0–39.0%) experienced multivessel spasm after infusion of a provocative agent. However, results varied widely in different series ranging from 0 to 100% (Table 2). Considering only the reports from Japan, where vasospastic ischaemia may be more common, the presence of spontaneous and provoked coronary spasm was also relatively uncommon (1.8 and 27.7%, respectively).
Abe et al.21 evaluated the coronary microcirculation using Doppler guidewire or contrast echocardiography. Although based only on few patients, their findings suggest that abnormalities in the coronary microcirculation do not contribute significantly to the syndrome. In contrast, Kurisu et al.13 found that the TIMI frame count, a validated index of coronary blood flow,24 was significantly higher in 28 patients with transient LV apical ballooning syndrome when compared with controls both during acute phase and follow-up. Bybee et al.12 confirmed these findings, evaluating coronary angiograms on admission. They also evaluated the TIMI frame counts in 16 patients with this syndrome, who were compared with 16 age- and gender-matched controls, without coronary atherosclerosis but who underwent coronary angiography before valve surgery. They found that all patients with transient LV apical ballooning syndrome had significantly abnormal TIMI frame counts in one or more epicardial coronary vessels; 10 patients had significantly abnormal TIMI frame counts in the distribution of all three major epicardial vessels and that mean TIMI frame counts were significantly higher compared with controls. These investigators interpreted their findings as indicative of diffuse coronary microvascular dysfunction and suggested that this abnormality may play a significant role in the pathogenesis of this syndrome. However, it remains unclear whether microvascular dysfunction is the primary cause of the syndrome or a secondary phenomenon.
Plasma levels of catecholamines and their metabolites were measured in four studies,6,11,13,19 which found elevated norepinephrine concentrations in 26 of 35 evaluated patients (74.3%, 95% CI: 57.9–85.8%; range: 50–100%). For instance, Wittstein et al.6 compared plasma catecholamine concentrations in 13 patients with transient LV apical ballooning syndrome with seven controls hospitalized for acute MI with Killip class III on presentation. They found that catecholamines levels were two to three times higher in patients with transient LV apical ballooning syndrome.
Four studies evaluated myocardial perfusion using single photon emission computed tomography (SPECT).11,13,19,21 Results of these studies showed moderate or severe myocardial ischaemia. Thus, Abe et al.21 reported that 11 of 13 patients (85%), in whom resting technetium-99m tetrofosmin tomographic myocardial imaging was performed during the acute phase, had decreased radioisotope uptake at the LV apex. Ito et al.19 reported that myocardial perfusion assessed by the SPECT imaging was impaired immediately after hospital admission, but improved considerably at 3–5 days. They interpreted the nuclear imaging findings of decreased myocardial perfusion in the absence of obstructive coronary lesions as direct evidence for impaired coronary microcirculation as a causative mechanism of this syndrome.
Endomyocardial biopsy was performed in 15 patients in four studies,6,9,13,21 with no evidence of myocarditis in any. Furthermore, viral antibodies were negative in all patients evaluated.
Discussion
The transient LV apical ballooning syndrome is a new diagnostic entity with typical characteristics. It occurs most frequently in women over 50 years of age. Although originally reported in Japan, it has been recently described in white Caucasians in Europe and North America. Almost 90% of reported patients are female and only few were younger than 50 years of age. The reason for the much more common occurrence in post-menopausal women is unclear. Several explanations have been proposed. Thus, sex hormones may exert important influences on the sympathetic neurohormonal axis25 and on coronary vasoreactivity.26 Women appear also to be more vulnerable to sympathetically mediated myocardial stunning,27 and post-menopausal alteration of endothelial function in response to reduced estrogen levels has been advocated as a possible alternative explanation.28
Patients suffering from transient LV apical ballooning syndrome may have a clinical presentation very similar to that of an ACS. Indeed, most patients present with chest pain or dyspnoea. Usually, the electrocardiogram at presentation shows ST-segment elevation and/or T wave inversion and pathological Q waves are present in almost 40% of patients. Furthermore, cardiac biomarker levels are frequently raised, mimicking acute MI, and LV function is impaired with regional wall-motion abnormalities. However, coronary angiography is completely normal in most patients and shows mild, non-obstructive coronary lesions (<50% luminal diameter stenosis). Differentiating transient LV apical ballooning syndrome from acute MI is important, as misdiagnosis may result in treatment with thrombolytic agents and may pose patients at unnecessary risk of bleeding. As opposed to patients with acute MI, those with transient LV apical ballooning syndrome have generally a benign prognosis. Only 1.1% of reported patients died during the hospitalization period and almost all surviving patients recovered fully. Unfortunately, with the exception of coronary angiography performed in the acute setting, there are no clinical, radiological, or laboratory characteristics that allow clinicians to diagnose this syndrome with certainty and to withhold urgent reperfusion therapy. However, when presented with post-menopausal women with a history of recent unusual emotional or physical stress, with relatively few traditional risk factors for coronary heart disease, and with the typical profound wall-motion abnormalities characteristic for this syndrome, urgent coronary angiography should be considered as an alternative to thrombolysis (Figure 2).
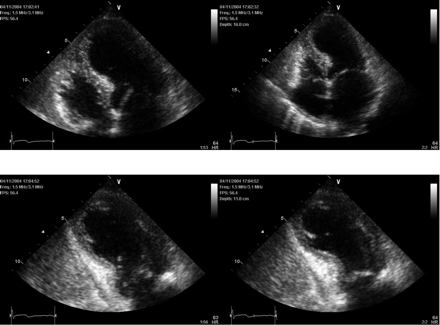
End-diastolic and end-systolic apical four-and-two chamber echocardiographic views demonstrating the typical apical and mid-ventricular LV wall-motion abnormalities of a patient with takotsubo cardiomyopathy diagnosed and managed at the McMaster University in Hamilton, Ontario.
Because the onset of this syndrome is often preceded by emotional or physical stress, catecholamine-mediated multivessel epicardial spasm,13 microvascular coronary spasm,21 or possible direct catecholamine-mediated myocyte injury29 have been advocated as possible pathophysiological mechanisms. However, the evidence supporting any of these possible mechanisms is not compelling. Spontaneous multivessel epicardial spasm is uncommon and after ergonovine or acetylcholine infusion, it was described in <30% of patients. Vasospastic ischaemia is much more common in Japan than the rest of the world. However, in our study, the prevalence of spontaneous and provoked coronary spasm was similar in Japanese and non-Japanese population. The two studies that evaluated the coronary microcirculation reported conflicting results. Elevated plasma catecholamines levels were found in almost 75% of the evaluated patients. However, in the absence of prospective data evaluating plasma catecholamine levels prior to the development of clinical abnormalities, a causal relationship cannot be proven and the elevated catecholamine levels may be an epiphenomenon or a consequence of the haemodynamic abnormalities associated with this syndrome. Myocarditis has been proposed as a possible alternative mechanism. However, studies that have used endomyocardial biopsy and viral serology do not support this hypothesis.6,21
Mid-ventricular wall-motion abnormalities, apical akinesia or dyskinesia with preserved or hyperkinetic contractile function of the basal LV segments, are characteristic. Local release of catecholamines from cardiac sympathetic efferent neurons seems to be an unlikely explanation because of the higher norepinephrine content and greater density of sympathetic nerves at the base of the heart when compared with the apex.30 However, several case reports of patients with pheochromocytoma-related cardiomyopathy described a similar distribution of LV wall-motion abnormalities.31 Furthermore, there is some evidence suggesting that the apical myocardium may be more responsive to sympathetic stimulation and may be more vulnerable to sudden catecholamine surges.32 A longitudinal, base-to-apex decline in LV myocardial perfusion, as described in patients with coronary risk factors, was also proposed as a possible alternative explanation.33
In the absence of studies specifically evaluating different therapies, the treatment of this syndrome remains entirely empirical and should be individualized according to the patient characteristics at the time of presentation. Standard supportive care with diuretics and vasodilators seems reasonable. Beta-agonists should be generally avoided and mechanical support seems to be preferable in patients with haemodynamic instability.
Our study has certain limitations. First, all data in our systematic review are derived from observational studies and bias in observational research cannot be excluded. Secondly, despite careful electronic and manual searches, we have probably not captured all cases of transient LV apical ballooning syndrome due to investigator and publication bias and our review is based on a relatively small number of patients and studies. Thirdly, some reported characteristics of this syndrome varied considerably among various studies. However, our systematic review represents the most complete review of the available data on this syndrome to date.
In conclusion, clinicians should be aware of the existence and the typical clinical manifestations of this syndrome, which is increasingly recognized in various populations. In particular, clinicians should consider this syndrome in the differential diagnosis of patients presenting with clinical findings suggestive of an ACS, especially in post-menopausal women with a recent history of acute emotional or physical stress.
Many questions regarding the aetiology, pathophysiology, and management of this syndrome remain unanswered and further research is needed to clarify these issues.
Conflict of interest: none declared.
References
- norepinephrine
- ventricular function, left
- ventricular fibrillation
- chest pain
- st segment elevation
- electrocardiogram q waves test
- t wave feature
- dyspnea
- cardiogenic shock
- left ventricle
- emotions
- differential diagnosis
- hospital mortality
- infarction
- medline
- postmenopause
- stress
- ejection fraction
- cardiac markers
- takotsubo cardiomyopathy
- symptom onset
- embase