Abstract
Ketoconazole is a nonselective steroid 17α-hydroxylase/17,20 lyase (CYP17A1) inhibitor that has been used, off-label, as a second-line therapy for castration-resistant prostate cancer (CRPC). The drug has shown clinical efficacy without survival benefit. Despite not improving survival, ketoconazole has beneficial characteristics, such as its low cost, a relatively favourable toxicity profile compared with chemotherapy, and its efficacy both before and after chemotherapy. The approval of several new, highly effective treatments, including abiraterone acetate, enzalutamide, and apalutamide, warrants re-evaluation of the role of ketoconazole and other classic agents in achieving the optimal timing and sequencing of available agents to prolong survival and maintain patients’ quality of life. In the current CRPC treatment landscape, we believe that ketoconazole can be considered in patients with nonmetastatic CRPC and in those with metastatic CRPC who do not respond to, tolerate, or have access to chemotherapy and other standard therapeutic options.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



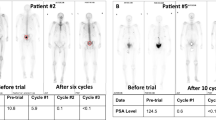
Figure adapted from ref.87, BMJ.
Similar content being viewed by others
References
National Cancer Institute. SEER Cancer Stat Fact Sheets: Prostate Cancer. National Institutes of Health https://seer.cancer.gov/statfacts/html/prost.html (2018).
Huggins, C. & H. C. Studies in Prostate Cancer. Cancer Res. 1, 293–297 (1941).
Loblaw, D. A. et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J. Clin. Oncol. 25, 1596–1605 (2007).
Borgmann, V., Hardt, W., Schmidt-Gollwitzer, M., Adenauer, H. & Nagel, R. Sustained suppression of testosterone production by the luteinising-hormone releasing-hormone agonist buserelin in patients with advanced prostate carcinoma. A new therapeutic approach? Lancet 1, 1097–1099 (1982).
Sharifi, N., Gulley, J. L. & Dahut, W. L. Androgen deprivation therapy for prostate cancer. JAMA 294, 238–244 (2005).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Locke, J. A. et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 68, 6407–6415 (2008).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Smith, M. R. et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 378, 1408–1418 (2018).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Fizazi, K. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 13, 983–992 (2012).
Fizazi, K. et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 377, 352–360 (2017).
Kucuk, O. et al. Phase II trial of bicalutamide in patients with advanced prostate cancer in whom conventional hormonal therapy failed: a Southwest Oncology Group study (SWOG 9235). Urology 58, 53–58 (2001).
Trachtenberg, J. & Pont, A. Ketoconazole therapy for advanced prostate cancer. Lancet 2, 433–435 (1984).
Taplin, M. E. et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin. Cancer Res. 15, 7099–7105 (2009).
Ang, J. E., Olmos, D. & de Bono, J. S. CYP17 blockade by abiraterone: further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br. J. Cancer 100, 671–675 (2009).
Yap, T. A., Carden, C. P., Attard, G. & de Bono, J. S. Targeting CYP17: established and novel approaches in prostate cancer. Curr. Opin. Pharmacol. 8, 449–457 (2008).
Small, E. J. et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J. Clin. Oncol. 22, 1025–1033 (2004).
Ryan, C. J. et al. Phase II study of ketoconazole plus granulocyte-macrophage colony-stimulating factor for prostate cancer: effect of extent of disease on outcome. J. Urol. 178, 2372–2376; discussion 2377 (2007).
Small, E. J., Baron, A. D., Fippin, L. & Apodaca, D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J. Urol. 157, 1204–1207 (1997).
Scholz, M. et al. Long-term outcome for men with androgen independent prostate cancer treated with ketoconazole and hydrocortisone. J. Urol. 173, 1947–1952 (2005).
Ryan, C. J. et al. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin. Cancer Res. 13, 2030–2037 (2007).
Keizman, D., Huang, P., Carducci, M. A. & Eisenberger, M. A. Contemporary experience with ketoconazole in patients with metastatic castration-resistant prostate cancer: clinical factors associated with PSA response and disease progression. Prostate 72, 461–467 (2012).
Eichenberger, T. & Trachtenberg, J. Effects of high-dose ketoconazole on patients who have androgen-independent prostatic cancer. Can. J. Surg. 32, 349–352 (1989).
Pont, A. Long-term experience with high dose ketoconazole therapy in patients with stage D2 prostatic carcinoma. J. Urol. 137, 902–904 (1987).
Witjes, F. J., Debruyne, F. M., Fernandez del Moral, P. & Geboers, A. D. Ketoconazole high dose in management of hormonally pretreated patients with progressive metastatic prostate cancer. Dutch South-Eastern Urological Cooperative Group. Urology 33, 411–415 (1989).
DeFelice, R., Johnson, D. G. & Galgiani, J. N. Gynecomastia with ketoconazole. Antimicrob. Agents Chemother. 19, 1073–1074 (1981).
Pont, A. et al. Ketoconazole blocks testosterone synthesis. Arch. Intern. Med. 142, 2137–2140 (1982).
Pont, A. et al. Ketoconazole blocks adrenal steroid synthesis. Ann. Intern. Med. 97, 370–372 (1982).
Bruno, R. D. & Njar, V. C. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg. Med. Chem. 15, 5047–5060 (2007).
Attard, G., Belldegrun, A. S. & de Bono, J. S. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 96, 1241–1246 (2005).
Sonino, N. The use of ketoconazole as an inhibitor of steroid production. N. Engl. J. Med. 317, 812–818 (1987).
Santen, R. J., Van den Bossche, H., Symoens, J., Brugmans, J. & DeCoster, R. Site of action of low dose ketoconazole on androgen biosynthesis in men. J. Clin. Endocrinol. Metab. 57, 732–736 (1983).
Suzuki, K. et al. Importance of the intracrine metabolism of adrenal androgens in androgen-dependent prostate cancer. Prostate Cancer Prostat. Diseases 10, 301–306 (2007).
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005).
Nelson, P. S. et al. Comprehensive analyses of prostate gene expression: convergence of expressed sequence tag databases, transcript profiling and proteomics. Electrophoresis 21, 1823–1831 (2000).
Almassi, N. et al. HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. 4, 554–557 (2017).
Hearn, J. W. D. et al. Association of HSD3B1 genotype with response to androgen-deprivation therapy for biochemical recurrence after radiotherapy for localized prostate cancer. JAMA Oncol. 4, 558–562 (2017).
Figg, W. D. et al. Prostate specific antigen decline following the discontinuation of flutamide in patients with stage D2 prostate cancer. Am. J. Med. 98, 412–414 (1995).
Scher, H. I. & Kelly, W. K. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J. Clin. Oncol. 11, 1566–1572 (1993).
Small, E. J. & Srinivas, S. The antiandrogen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer 76, 1428–1434 (1995).
Kantoff, P. W. et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J. Clin. Oncol. 17, 2506–2513 (1999).
Stanbrough, M. et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 66, 2815–2825 (2006).
Rittmaster, R., Hahn, R. G., Ray, P., Shannon, J. B. & Wurzel, R. Effect of dutasteride on intraprostatic androgen levels in men with benign prostatic hyperplasia or prostate cancer. Urology 72, 808–812 (2008).
Thomas, L. N. et al. Type 1 and type 2 5α-reductase expression in the development and progression of prostate cancer. Eur. Urol. 53, 244–252 (2008).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Ernst, D. S. et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J. Clin. Oncol. 21, 3335–3342 (2003).
Berry, W., Dakhil, S., Modiano, M., Gregurich, M. & Asmar, L. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J. Urol. 168, 2439–2443 (2002).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Sweeney, C. J. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
James, N. D. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016).
Zhu, M. L. et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 70, 7992–8002 (2010).
Gan, L. et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 69, 8386–8394 (2009).
Kim, W. et al. Sequential use of the androgen synthesis inhibitors ketoconazole and abiraterone acetate in castration-resistant prostate cancer and the predictive value of circulating androgens. Clin. Cancer Res. 20, 6269–6276 (2014).
Mezynski, J. et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann. Oncol. 23, 2943–2947 (2012).
Aggarwal, R. et al. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance). Cancer 119, 3636–3643 (2013).
Pond, G. R. et al. Efficacy of docetaxel-based chemotherapy following ketoconazole in metastatic castration-resistant prostate cancer: implications for prior therapy in clinical trials. Urol. Oncol. 31, 1457–1463 (2013).
Nakabayashi, M. et al. Activity of ketoconazole after taxane-based chemotherapy in castration-resistant prostate cancer. BJU Int. 105, 1392–1396 (2010).
Dreicer, R., See, W. A. & Klein, E. A. Phase II trial of GM-CSF in advanced prostate cancer. Investigat. Drugs 19, 261–265 (2001).
Amato, R. J., Saxena, S. & Stepankiw, M. Phase II trial assessing granulocyte-macrophage—colony stimulating factor, ketoconazole plus mitoxantrone in metastatic castration-resistant prostate cancer progressing after docetaxel treatments. Cancer Invest. 31, 177–182 (2013).
Gil-Bazo, I. et al. Safety and efficacy of maintenance therapy with a nonspecific cytochrome P17 inhibitor (CYP17i) after response/stabilization to docetaxel in metastatic castration-resistant prostate cancer. Clin. Genitourinary Cancer 11, 78–84 (2013).
Van Veldhuizen, P. J. et al. Docetaxel and ketoconazole in advanced hormone-refractory prostate carcinoma: a phase I and pharmacokinetic study. Cancer 98, 1855–1862 (2003).
Figg, W. D. et al. A phase I clinical study of high dose ketoconazole plus weekly docetaxel for metastatic castration resistant prostate cancer. J. Urol. 183, 2219–2226 (2010).
Trump, D. L. et al. High-dose ketoconazole in advanced hormone-refractory prostate cancer: endocrinologic and clinical effects. J. Clin. Oncol. 7, 1093–1098 (1989).
Nakabayashi, M. et al. Response to low-dose ketoconazole and subsequent dose escalation to high-dose ketoconazole in patients with androgen-independent prostate cancer. Cancer 107, 975–981 (2006).
Lo, E. N. et al. Prospective evaluation of low-dose ketoconazole plus hydrocortisone in docetaxel pre-treated castration-resistant prostate cancer patients. Prostate Cancer Prostat. Diseases 18, 144–148 (2015).
Barrie, S. E. et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J. Steroid Biochem. Mol. Biol. 50, 267–273 (1994).
Potter, G. A., Barrie, S. E., Jarman, M. & Rowlands, M. G. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J. Med. Chem. 38, 2463–2471 (1995).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Danila, D. C. et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol. 28, 1496–1501 (2010).
Leibowitz-Amit, R. et al. Abiraterone acetate in metastatic castration-resistant prostate cancer: a retrospective review of the Princess Margaret experience of (I) low dose abiraterone and (II) prior ketoconazole. Eur. J. Cancer 50, 2399–2407 (2014).
Peer, A. et al. Comparison of abiraterone acetate versus ketoconazole in patients with metastatic castration resistant prostate cancer refractory to docetaxel. Prostate 74, 433–440 (2014).
McKay, R. R. et al. A Phase II trial of abiraterone combined with dutasteride for men with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 23, 935–945 (2017).
Basch, E. et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J. Clin. Oncol. 32, 3436–3448 (2014).
Norum, J. & Nieder, C. Treatments for metastatic prostate cancer (mPC): a review of costing evidence. PharmacoEconomics 35, 1223–1236 (2017).
Guirgis, H. M. The value of anticancer drugs in metastatic castrate-resistant prostate cancer: economic tools for the community oncologist. J. Commun. Supportive Oncol. 13, 362–366 (2015).
Cornford, P. et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 71, 630–642 (2017).
Cookson, M. S., Lowrance, W. T., Murad, M. H. & Kibel, A. S. American Urological Association. Castration-resistant prostate cancer: AUA guideline amendment. J. Urol. 193, 491–499 (2015).
Mohler, J. L. et al. Prostate cancer, Version 1.2016. J. Natl Compr. Canc. Netw. 14, 19–30 (2016).
Virgo, K. S. et al. Second-line hormonal therapy for men with chemotherapy-naive, castration-resistant prostate cancer: American Society of Clinical Oncology provisional clinical opinion. J. Clin. Oncol. 35, 1952–1964 (2017).
Ryan, C. J. et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 16, 152–160 (2015).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Beer, T. M. & Tombal, B. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 1755–1756 (2014).
Ritch, C. R. & Cookson, M. S. Advances in the management of castration resistant prostate cancer. BMJ 355, i4405 (2016).
Reviewer information
Nature Reviews Urology thanks M. Carducci and W. Figg for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
V.P. and B.L. researched data and made substantial contributions to discussion of the content of the article. All authors wrote and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, V., Liaw, B. & Oh, W. The role of ketoconazole in current prostate cancer care. Nat Rev Urol 15, 643–651 (2018). https://doi.org/10.1038/s41585-018-0077-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-018-0077-y