Abstract
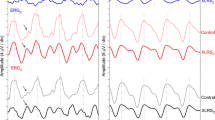
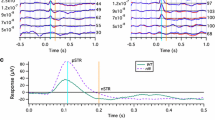
Intensity-series rod and cone ERGs were recorded in 19 patients with XLRS and 26 control eyes. All patients were examined by one ophthalmologist and diagnosed on the basis of fundus appearance and evidence of the disease in other males in the same family. Mutations in the XLRS1 gene have been identified in 15 of the patients. Dark-adapted ERGs were significantly different from controls for all test conditions and for both a-wave and b-wave responses. Abnormalities were detectable in all patients but there was considerable variation in the severity of abnormality. One third of the patients had the dark-adapted `negative-wave' response typically associated with inner retinal disorder, but about one third showed only mild depression of the b-wave while the remainder had abnormally low a-waves in addition to depressed b-waves. Light-adapted responses were also affected and both a-wave and b-wave responses differed significantly from controls, but the `negative-wave' response was not seen in any patient. The severity of the ERG abnormality did not correlate with the classification of fundus appearance or patient age suggesting that retinal function is relatively stable throughout life. The severity of ERG abnormalities did not correlate with the type of mutation and responses could differ between affected males within the same family. These results indicate considerable heterogeneity of ERG response without clinical, age or genetic correlate. The abnormal a-wave responses indicate that photoreceptor as well as inner retinal layer function may be affected in XLRS, at least in some patients.
Similar content being viewed by others
References
Deutman AF. Sex-linked juvenile retinoschisis. In: Deutman AF, ed. The hereditary dystrophies of the posterior pole of the eye. Assen, The Netherlands: Van Gorcum, 1971: 48–98.
George NDL, Yates JRW, Moore AT. Clinical features in affected males with retinoschisis. Arch Ophthalmol 1996; 114: 274–80.
George NDL Yates JRW, Moore AT. X-linked retinoschisis. Br J Ophthalmol 1995; 79: 697–702.
Forsius H, Kraus U, Helve J, Vuopala V, Mustonen, E, Vainio-Mattila B. Visual acuity in 183 cases of X-chromasomal retinoschisis. Can J Ophthalmol 1973; 3: 385–93.
Kellner U, Brummer 5, Foerster MH, Wessing A. X-linked congenital retinoschisis. Graefe's Arch Clin Exp Ophthalmol 1990; 228: 432–7.
Sieving PA, Bingham EL, Roth MS, Young MR, Boehnke M, Kuo C, Ginsburg D. Linkage relationship of X-linked retinoschisis with Xp22.1-22.3 probes. Am J Hum Genet 1990; 47: 616–21.
George NDL, Payne SJ, Bill RM, Barton DE, Moore AT, Yates JRW. Improved genetic mapping of X-linked retinoschisis. J Med Genet 1995; 33: 918–22.
Walpole SM, Nicolaou A, Howell GR, Whittaker A, Bentley DR, Ross MT, Yates JRW, Trump D. High resolution physical map of the X-linked retinoschisis interval in Xp22. Genomics 1997; 44: 300–8.
Sauer CG, Gehrig A, Warneke-Wittstock R, Marquardt A, Ewing CC, Gibson A, Lorenz B, Jurklies B, Weber BHF. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet 1997; 17: 164–70.
The Retinoschisis Consortium. Functional implications of the spectrum of mutations found 234 cases with X-linked juvenile retinoschisis (XLRS 1). Hum Mol Genet 1998; 7: 1185–92.
Peachey NS, Fishman GA, Derlacki DJ, Brigell MG. Psychophysical and electroretinographic findings in X-linked juvenile retinoschisis. Arch Ophthalmol 1987; 105: 513–6.
Papakostopoulos D, Doran RML, Ragge NK. The electrooculogram, electroretinogram and pattern-reversal visual evoked potentials in juvenile X-linked retinoschisis. Zdrav Vestn 1993; 62: 79–84.
George NDL, Yates JRW, Bradshaw K, Moore AT. Infantile presentation of X-linked retinoschisis. Br J Ophthalmol 199S; 79: 653–7.
Miyake y, Shiroyama N, Ota I, Horiguchi M. Focal macular electroretinogram in Xlinked retinoschisis. Invest Ophthalmol Vis Sci 1993; 34: 512–5.
Hood DC, Birch DG. B-wave of the scotopic (rod) electroretinogram as a measure of the activity of human on-bipolar cells. J Opt Soc Am 1996; 13: 623–33.
Muruyama K, Kuo CY, Sieving PA. Abnormal threshold ERG responses in X-linked juvenile retinoschisis: evidence for a proximal retinal origin of the human STR. Clin Vision Sci 1991; 6: 317–22.
Condon GP, Brownstein 5, Wang N, Kearns AF, Ewing CC. Congenital hereditary (juvenile X-linked) retinoschisis: histopathologic and ultrastructural findings in three eyes. Arch Ophthalmol 1986; 104: 576–83.
Marmor MF, Zrenner E. Standard for clinical electroretinography (1994 update). Doc Ophthalmol 1995; 89: 199–210.
Hood DC, Birch DG. Computational models of rod-driven retinal activity. IEEE Eng Med Biol Mag 1995; 14: 59–66.
Jacobi PC, Miliczek K-D, Zrenner E. Experience with the international standard for clinical electrophysiology: normative values for clinical practice, interindividual and intraindividual variations and possible extensions. Doc Ophthalmol 1993; 85: 95–114.
Hood DC, Birch DG. Assessing abnormal rod photoreceptor activity with the a-wave of the electroretinogram: applications and methods. Doc Ophthalmol 1997; 92: 253–67.
Breton ME, Schuller AW, Lamb TD, Pugh EN. Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci 1994; 35: 295–309.
Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci 1992; 8: 107–26.
Trump D, Grayson C, Sowden J, Ellis J, Bobrow LG, Yates JRW. X-Linked Retinoschisis: Investigation of the XLRS1 expression pattern and characterisation of a novel antibody against the XLRS1 protein. Invest Ophthalmol Vis Sci 1999; 40: S961.
Rights and permissions
About this article
Cite this article
Bradshaw, K., George, N., Moore, A. et al. Mutations of the XLRS1 gene cause abnormalities of photoreceptor as well as inner retinal responses of the ERG. Doc Ophthalmol 98, 153–173 (1999). https://doi.org/10.1023/A:1002432919073
Issue Date:
DOI: https://doi.org/10.1023/A:1002432919073