Abstract
Pertussis is a potentially severe respiratory disease, which affects all age groups from young infants to older adults and is responsible for an estimated 195,000 deaths occurred globally in 2008. Active research is ongoing to better understand the pathogenesis, immunology, and diagnosis of pertussis. For diagnosis, molecular assays (e.g., polymerase chain reaction) for detection of Bordetella pertussis have become more widely available and support improved outbreak detection. In children, pertussis vaccines have been incorporated into routine immunization schedules and deployed for pertussis outbreak control. Lower levels of vaccine coverage are now being observed in communities where vaccine hesitancy is rising. Additionally, recognition that newborn babies are at risk of pertussis in the USA and UK has led to recommendations to immunize pregnant women. Among adolescents and older adults in the USA, Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular pertussis (Tdap) Vaccines are recommended, but substantial individual- and system-level barriers exist that will make achieving national Healthy People 2020 targets for immunization challenging. Current antimicrobial regimens for pertussis are focused on reducing the severity of disease, reducing rates of sequelae, and minimizing transmission of infection to susceptible individuals. Continued surveillance for pertussis will be important to identify opportunities for reducing young infants’ exposure and reducing the impact of outbreaks among school-aged children. Laboratory-based surveillance for newly emerging strains of B. pertussis will be important to identify strains that may evade protection elicited by currently available vaccines. Efforts to develop new-generation pertussis vaccines should be considered now in anticipation of vaccine development programs, which may require ten or more years to deliver a licensed vaccine.
Similar content being viewed by others
Pertussis should be considered a serious and potentially deadly infectious disease in infants. |
Although vaccines for pertussis are widely available for all ages, low vaccine uptake among pregnant women and other adults is a continued problem. |
Given currently available vaccines, future research to enhance vaccine effectiveness and the duration of protection should be encouraged. |
Recent increases in pertussis outbreaks suggest the need for increased vaccine advocacy and education, particularly in affected communities that may be at continued risk. |
1 Introduction and Clinical Presentation
Pertussis—also known as “whooping cough”—is caused by the Gram-negative coccobacilli bacteria Bordetella pertussis [1]. Pertussis has ancient roots from the sixth century, when it was described as the “the cough of 100 days” in China [2]. In the early nineteenth century, Jules Bordet and Octave Gengou isolated B. pertussis from a sputum sample from Bordet’s son when he had a cough illness [3]. In its clinical course, the classical progression of pertussis can be divided into three consecutive stages (the catarrhal, paroxysmal, and convalescent stages). Each stage may last for up to 3 weeks, and, typically, full recovery from the illness occurs in 2–3 months [4–7]. In the catarrhal stage, the pertussis diagnosis is often missed because of the presence of signs and symptoms that overlap with viral upper respiratory tract infections. Healthcare professionals, including pediatricians and primary care providers, need to maintain a high index of suspicion for pertussis when they encounter patients in whom a persistent cough is the presenting complaint [5]. In the paroxysmal stage, patients usually experience intense cough episodes, which may last for several minutes. Cough paroxysms are usually associated with inspiratory whoops, bluish discoloration of mucous membranes, eye bulging, tongue protrusion, excessive salivation, and watery eyes. Soon after each cough episode, patient may also experience vomiting, fatigue, and respiratory exhaustion [8]. In the convalescence stage, the cough pattern decreases in its intensity and duration, but a milder form of cough may remain for up to 6 weeks. However, in recovering children, cough paroxysms similar to pertussis may recur if a child becomes sick with a respiratory viral infection [5, 6, 9, 10].
For families, health systems, and society, the impact of pertussis is immediate, substantial, and attended by long-term consequences. Health outcomes associated with pertussis range from mild clinical illness (which may be treated in outpatient settings) to severe disease (requiring intensive care unit support), as well as complications that result in death. In older children, adolescents, and adults, signs and symptoms of pertussis may be overlooked [11–14]. In neonates and young infants, who may be too young to complete the primary series of pertussis vaccine, such missed diagnoses may have devastating clinical consequences [15]. In infants and newborns who develop complications of pertussis, the most common findings that are observed include apnea, high rates of hospitalization, pneumonia, weight loss, otitis media, convulsions, and death [16]. The aim of this review is to provide up-to-date information about the disease epidemiology, available diagnostic tools, prevention, post-exposure prophylaxis (PEP), and treatment of pertussis.
2 Epidemiology and Disease Burden of Pertussis
2.1 Global Burden of Pertussis
Pertussis is a disease with a global distribution and remains one of the leading causes of vaccine-preventable deaths [17]. On the basis of 2008 disease burden estimates (the most recent year for which data are available) from the World Health Organization (WHO), pertussis is responsible for ~16 million cases and 195,000 deaths worldwide. Approximately 43 % of reported pertussis deaths have occurred in Africa [18]. The lack of national data on pertussis from laboratory-based surveillance systems in many countries has led to challenges in maintaining current global disease burden estimates.
2.2 Pertussis Burden in the USA
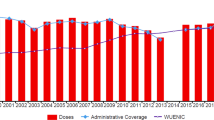
Over the past two decades in the USA, pertussis has been the most commonly reported vaccine-preventable disease associated with severe morbidity and mortality among infants [19–24]. For the last several years, the nationwide incidence of pertussis has dramatically increased in a wide range of ages, with neonates and young infants experiencing the highest rates of illness and sequelae (Fig. 1). In 2012, the incidence of pertussis was approximately 127/100,000 for infants <1 year of age, with a 15 % case-fatality rate reported among infants <3 months of age [25]. In 2013, the rates of pertussis were approximately 160 and 45 per 100,000 for infants aged <6 months and those aged 6–11 months, respectively, with 12 deaths reported among infants <3 months of age [26]. In 2014, the incidence of pertussis among infants <6 months of age was approximately 151/100,000 infants, and for those aged 6–11 months, the incidence rate was 40/100,000 infants. In 2014, a total of seven deaths were reported among infants <3 months of age [27]. The rates of infant hospitalizations associated with pertussis far exceed those observed in older age groups. For instance, previous reports showed that ~50 % of infants with pertussis require hospitalization, whereas the hospitalization rate among adolescents and adults was ~6 % [16, 28, 29]. A large proportion of hospitalized infants require intensive care and respiratory support [16].
Like other countries, the USA has experienced a number of pertussis outbreaks over the last two decades. The pertussis resurgence has been linked to many factors. One of these factors is the high vaccination exemption rates across the country, where many states offer nonmedical waivers for school requirements. These include exemptions for religious and parent personal beliefs [30–32]. In the last 5 years, at least five large pertussis outbreaks have been reported from geographically diverse areas, including California, Michigan, Minnesota, Ohio, and Washington. In 2012, Washington State experienced a large outbreak, in which most county jurisdictions were affected, and officials reported a total of 4918 confirmed and probable cases, for an overall incidence of ~11/100,000 [33, 34]. In this outbreak, the incidence among infants was 107/100,000. Among a total of 95 confirmed infant pertussis cases, 35 infants required hospitalization [33]. During the California pertussis outbreak of 2014, state officials reported a record number of cases (n = 10,831) [35]. Among the reported cases, 376 required hospitalization and 227 cases (60 %) were identified among infants <4 months of age. Eighty-five infants (23 %) required intensive care. Four infants <2 months of age died.
On the basis of routinely reported pertussis surveillance data from state health departments, several areas in the USA reported unusually high numbers of pertussis cases to the US Centers for Disease Control and Prevention (CDC) during the period from 2009 to 2014. In total, health departments reported 26,566 pertussis cases from California [35], 3038 from Michigan [36], 4144 from Minnesota [37], 2958 from Ohio [38], and 6231 Washington [33].
2.3 Pertussis Burden in Europe
In the European Union (EU) and associated countries, pertussis data from 2012 have shown an overall incidence rate of ~11/100,000, and the number of pertussis cases has more than doubled in comparison with previous years (2008–2011) (Table 1; Fig. 2). In 2012, 38,840 cases were reported by 28 EU/European Economic Area (EEA) countries and, of these countries, 26 have national surveillance systems that capture pertussis reports. Although comparison between countries should be done only with caution because of variations between surveillance systems and different degrees of awareness in the reporting of the disease, the highest incidence rates of pertussis were reported from Norway (85.2/100,000), the Netherlands (76.9/100,000), Denmark (20.4/100,000), the UK (19/100,000), and Slovakia (17/100,000) [15].
Distribution of confirmed pertussis cases by month in the European Union/European Economic Area (EU/EEA), 2008 through 2012. Reproduced with permission [15]
In the same European Center for Disease Prevention and Control (ECDC) report of 2012 pertussis data, confirmed cases of pertussis were reported most frequently among young children and adolescents aged 5–14 years, in whom a relatively higher incidence rate of 23.7/100,000 was noted. The higher incidence of pertussis in this age group has been attributed to the fact that Norway and the Netherlands had the highest overall notification rates, with particularly high rates among children aged 5–14 years. However, in several other European countries, children aged <5 years experienced the highest incidence rates of pertussis (23.6/100,000 population). Overall, females had a slightly higher incidence rate of pertussis, with a female to male case ratio of 1:0.86.
2.4 Pertussis Burden in Other Countries
In other regions of the world, pertussis continues to threaten infants and children. From 2011 through 2013, a large pertussis outbreak struck Auckland, New Zealand, and led to 62 confirmed pediatric intensive care unit (PICU) pertussis hospitalizations among children aged 2 weeks through 3 years, and 98 % of the children admitted to the PICU were <6 months of age [39]. In these New Zealand children with severe pertussis, the mean length of stay was 12 days, resulting in 783 patient-days for these hospitalized children. In that hospital, a total of six deaths were reported from 2003 to 2013, with three occurring from 2011 to 2013. The total cost of the 2011–2013 pertussis outbreak was ~NZ$3 million, and the estimated annual cost of pertussis management in the PICU was ~NZ$82,000.
In many countries, such as South Korea and Italy, there is significant underreporting of pertussis in established disease surveillance systems [40–42]. Previous studies have suggested that passive surveillance may result in an underestimation of the actual pertussis incidence by a factor of 10–1000 [43, 44]. In 2015, national surveillance reports from the China Center for Disease Control showed total figures of 1712 pertussis cases in 2013 and 3408 pertussis cases in 2014 from a total country of 1.364 billion residents, suggesting the presence of gross underreporting in China [45, 46]. A recent study in China implemented active surveillance to determine the incidence rates of pertussis over 2 years (2010–2012) in three communities with a total population of ~160,000 [41]. In this study, Huang and colleagues screened >1000 individuals with a clinical picture suggestive of pertussis. A total of 1022 nasopharyngeal (NP) swabs were tested by polymerase chain reaction (PCR), and 802 blood samples were tested for antibodies to B. pertussis toxin (PT). Of those tested, pertussis was confirmed in 113 individuals, showing an annual incidence of pertussis of 23.5/100,000 in this population. These results showed an incidence rate from active surveillance that was 16 times higher than the incidence observed during passive surveillance over the same time period. For individuals aged 15–69 years, the actual incidence rate of pertussis was ~43 times higher than reported hospital rates. Notably, 85 % of pertussis cases among children <15 years of age were reported among children who had been previously immunized with a primary series of pertussis-containing vaccine. Huang and others showed that only 5 % of pertussis cases were accurately diagnosed at their first clinic visit. This study in China underscores the challenge found in many countries, where pertussis may be underdiagnosed when cases initially present to clinical facilities [47, 48].
Several factors explaining the resurgence of pertussis have been suggested [49–56]. These include waning immunity following natural infection or vaccination; improved surveillance methods; improved availability of diagnostic tests (e.g., PCR), leading to increased case detection; increased awareness of pertussis among healthcare providers, leading to increased case reporting; incomplete pertussis immunization series, leading to breakthrough disease; and changes of antigenic structure and adaptation of B. pertussis, which may escape antibodies elicited by currently available vaccines. In 2010, King et al. conducted an analytic study using microarray-based comparative genomic hybridization to determine the gene content of B. pertussis strains in 171 B. pertussis isolates from Australia, Japan, Kenya, the Netherlands, Senegal, and Sweden, with different vaccination schedules between 1949 and 2008 [57]. The results of this study showed that B. pertussis is dynamic and is continuously evolving as a result of adaptive evolution.
3 Cost of Pertussis Illness
Starting in 2001, work by the Global Pertussis Initiative suggested that the direct and indirect costs associated with pertussis are substantial [58]. Direct costs associated with pertussis include charges associated with doctor visits, urgent care or emergency room visits, inpatient hospitalization, diagnostic laboratory tests, and radiologic tests, as well as therapeutic intervention costs. Indirect costs include school and work absences, reduced work productivity, and time away from work for parents who care for a sick child. The direct costs tend to be higher in infants than in other age groups because of higher rates of pertussis complications, which often require hospitalization or intensive care.
In 2000, Lee and colleagues estimated the economic burden of pertussis for 87 individuals among diverse age groups in 69 households [59]. This group found that the direct costs associated with pertussis averaged $2800 for infants, $300 for children, $250 for adolescents, and $180 for adults. In this study, indirect costs amounted to an average of $767 per family and an average of six lost work days. In a separate study from 2004, the average total cost of pertussis among adults was higher than that for children ($1950 versus $800) [60]. The direct costs associated with pertussis treatment were higher for adults ($330) than for children ($240), and the indirect costs associated with pertussis were 90 % higher for adults than for adolescents ($450 versus $155). In 2014, Lopez et al. found that pertussis-associated hospitalizations in young infants (aged 0–6 months) increased 53 % from $14,520 in 1997 to $22,278 in 2009 [61]. If severe neurologic sequelae of pertussis, such as encephalitis, are taken into account, the direct medical costs of pertussis may be much higher. In two Canadian studies, the direct medical costs for children with pertussis-associated encephalitis or chronic neurologic deficits were approximately $27,640 and $103,650 Canadian dollars, respectively [62, 63].
In 2014, McGarry et al. conducted a cost-effectiveness study to evaluate Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) vaccine introduction for individuals aged 65 years and older [64]. In their modeling, McGarry et al. showed that the costs of treating mild, moderate, and severe pertussis were ~$83–102, $203–607, and $6432–13,029, respectively. In this study, the cost of illness in infants (aged <1 year) with moderate to severe pertussis was almost double the cost in other age groups.
4 Laboratory Diagnosis
Early detection and diagnosis of pertussis relies on a high index of suspicion, collection of appropriate clinical laboratory specimens, and rapid application of available diagnostic tests for pertussis (Table 2). Delays in diagnosis and treatment of pertussis facilitate its transmission to household contacts, friends, schoolmates, and family members [65–67]. Although typical pertussis may be diagnosed on clinical grounds alone, the assessment of clinical manifestations may be unreliable particularly in patients who manifest atypical presentations that mimic other infections [68]. Culture-based diagnosis of B. pertussis is considered definitive and is assumed to have absolute specificity in association with an unexplained cough. In the past two decades, advances in laboratory diagnosis of pertussis have enabled earlier disease detection. For example, culture and direct fluorescent antibody staining of NP secretions have been largely replaced by nucleic acid amplification tests—notably, PCR [69].
PCR is a nucleic acid amplification test, which detects DNA sequences of B. pertussis in clinical respiratory specimens. PCR is becoming widely used in many clinical settings and provides a timely diagnostic tool that does not require viable organisms and can be used up to 4 weeks after the onset of a cough [70–73]. After the fourth week from cough onset, B. pertussis DNA diminishes in the nasopharynx, resulting in low PCR sensitivity and thus high false negative results [72]. Although the sensitivity of PCR is estimated to be 19 % higher than that of culture alone, PCR gives lower specificity and more frequent false positive results than culture [12, 70, 71, 74, 75]. False positive results can be due to several factors—most notably, contamination of specimens during sample collection with either the native organism or an amplicon created from a previous reaction [76]. Ideally, specimens should be collected and vaccines administered in separate areas, using a careful technique and decontamination of surfaces. It is also paramount to test only patients who are symptomatic [73]. To avoid errors in PCR detection of pertussis, the test should be used only in patients whose clinical presentation is suggestive of pertussis and before initiation of antibiotic treatment [72, 75]. In addition, when PCR is used to test asymptomatic close contacts for pertussis, the test result should not drive any clinical decision related to PEP [72].
In the last two decades, several enhancements of PCR assays have improved test specificity and performance. The test has changed substantially from agarose gel or microwell plate detection of amplicons to real-time amplification and detection [69]. Most PCR tests for pertussis are based on detection of an insertion sequence (IS)—a transposable DNA element approximately 1000 base pairs (bp) long, which has been inserted into multiple sites on the same chromosome. There are usually multiple copies of an IS per genome, which increases the sensitivity of PCR tests. IS481 is perhaps the most well described and validated target for B. pertussis [69], and for detecting Bordetella parapertussis, IS1001 is most commonly used [77–79]. There are >50 copies of IS481 per B. pertussis genome [80]. However, IS481 is also present in other Bordetella species, such as Bordetella holmesii and Bordetella bronchiseptica [80, 81].
To enhance the specificity of the test, PCR tests that target more than one IS have recently been developed and evaluated [71, 82]. In 2007, Qin et al. compared the use of a three-IS-targeted real-time PCR assay (IS481, PT ptxA promoter region, and outer membrane porin, or recA) versus a conventional single-target sequence (IS481) in respiratory specimens collected from >4000 patients with suspected pertussis [71]. In comparison with the single-target IS, the three-target combination increased the proportion of truly positively identified specimens by 1.25-fold, and two-target combinations increased the proportion of positively identified specimens by 1.10- to 1.24-fold. In 2013, Tizolova et al. screened the genomes of recently circulating isolates of Bordetella species by using PCR to compare the prevalence rates of IS481, IS1001, and IS1002 with previously published data, and to sequence all IS that were detected [83]. Tizolova and colleagues confirmed that targeting IS481 and IS1001 did not provide high test specificity, because multiple species were detected, including B. pertussis, B. holmesii, B. bronchiseptica (IS481), B. parapertussis, and B. bronchiseptica (IS1001).
Another PCR-based diagnostic tool, the FilmArray Respiratory Panel (RP), has also been developed and evaluated [84]. Although RP kits are appropriate for a core laboratory or point-of-care clinic because of their ease of use, they have less sensitivity than real-time PCR [84]. Jerris et al. recently compared the FilmArray RP and a focused B. pertussis PCR assay in 71 clinical specimens [84]. The RP targets the promoter region of the PT gene, whereas the focused real-time PCR assay targets IS481. They concluded that the FilmArray RP assay detects approximately one third fewer cases of B. pertussis than the focused PCR assay.
Although PCR is the nucleic acid amplification test most frequently used to detect B. pertussis, an assay based on loop-mediated isothermal amplification (LAMP) has also been developed [85–87]. LAMP is a relatively new, rapid method to amplify DNA, with high sensitivity and specificity, and has been developed and commercialized to target a number of pathogens, including B. pertussis. The method consists simply of incubating a mixture of the target gene, 4–6 different primers, Bst DNA polymerase, and substrates. The advantages of the LAMP method are that it has high amplification efficiency without the need for thermal cycling and therefore is less costly [85, 87–89].
The results from PCR testing should be considered in the context of clinical symptoms of pertussis to guide therapeutic decision-making [73]. To optimize diagnose and case detection, clinicians and public health staff should utilize standardized NP swab specimen collection methods, as described by the CDC [90]. Either an NP swab or NP aspirates are considered adequate for culture or PCR testing [90].
Serologic testing has high sensitivity for pertussis diagnosis, particularly at a late stage in the illness when the bacteria are no longer detectable [91]. Although serology is not a useful diagnostic tool in neonates or young infants [73], antibody titers could be of value for diagnosis in adolescents and adults, who usually seek medical advice late in the disease course when culture and PCR would be less reliable [92]. In addition, two serum specimens collected several weeks apart to demonstrate a fourfold or greater change in the immunoglobulin (Ig) G titer are required [69]. The WHO includes paired serology as part of the criteria for laboratory confirmation of pertussis.
Although serologic testing is done in many countries and is within the laboratory confirmation criteria of the WHO [93], standardized assays are not widely available in the USA and are less often used [73]. In Massachusetts, the state health department offers a positive single-point PT-specific IgG serology to support improved surveillance and confirmation of pertussis in individuals aged 11 years and older [28, 75, 94]. In states other than Massachusetts, cases meeting the clinical case definition that are seropositive but culture- or PCR-negative should be reported as probable cases [72].
The most meaningful serologic results are obtained using enzyme-linked immunosorbent assay (ELISA)-based formats with purified antigens, such as PT and filamentous hemagglutinin (FHA). Because PT is produced only by B. pertussis, detection of PT antibodies constitutes the most specific serologic indicator of pertussis infection [95–99]. Unfortunately, serologic diagnosis is complicated by the fact that PT is present in substantial amounts in all acellular vaccines. Therefore, antibodies generated by infection versus vaccination cannot be definitively distinguished for at least 6 months after vaccination [100]. Because of the continuous circulation of B. pertussis in the population, anti-PT IgG is likely detectable in most adolescent and adult populations, so it is necessary to establishing a cutoff value correlated with recent infection. Thus, accurate interpretation of serologic results requires knowledge of both the vaccination history and possible exposures to determine the reason for detectable antibody responses to B. pertussis [73].
5 Post-Exposure Prophylaxis and Treatment
Supportive care is the mainstay of management for B. pertussis and may include hospital admission for close monitoring of respiratory status and fluid or nutritional support. Furthermore, known triggers for coughing paroxysms (e.g., cold temperatures) should be avoided [101, 102]. In addition to supportive management, antimicrobial agents are recommended for prophylaxis of family members and other close contacts to help reduce the risk of secondary cases regardless of vaccination status [103]. The decision to prescribe antibiotics for close contacts should be made on a case-by-case basis. Factors that could influence this decision include the exposure duration and intensity, high-risk group membership (e.g., infants <1 year old), and the potential for developing a severe form of pertussis [6]. Macrolide antibiotics are recommended as chemoprophylaxis agents. For infants aged >1 month, children, and adults, erythromycin or clarithromycin are preferred agents. In neonates, azithromycin is the PEP drug of choice [104, 105].
Macrolide antibiotics are also considered first-line agents for the treatment of pertussis (Table 3) [105]. These antimicrobials are most effective when given early in the course of the disease—ideally, in the catarrhal stage [105, 106]. In addition to decreasing the severity of the disease, antimicrobial treatments are effective in clearing the nasopharynx of B. pertussis in symptomatic and asymptomatic individuals and consequently preventing the spread of the infection [107–109]. Erythromycin has been the antibiotic of choice for treatment of pertussis or PEP, but, because of potentially uncomfortable side effects (e.g., gastrointestinal upset), which result in poor adherence to the treatment regimen, the newer macrolides (i.e., clarithromycin and azithromycin) have been prescribed more often [105]. In 1999, Halperin et al. conducted a double-blind controlled trial to determine whether erythromycin for PEP is effective in household contacts [110]. Halperin and colleagues studied 152 culture-confirmed cases of pertussis in children, and the household contacts of those confirmed cases were randomly assigned to receive either erythromycin (40 mg/kg/day in three divided doses) or placebo for 10 days. It was found that erythromycin successfully eradicated B. pertussis in ~68 % of household contacts. In 2007, Altunaiji et al. conducted a Cochrane review of 13 clinical trials involving >2000 participants to assess the risks and benefits of antimicrobial treatment of, and PEP against, whooping cough in children and adults [107]. They found that short-term antibiotics (azithromycin for 3–5 days; clarithromycin or erythromycin for 7 days) were as effective as long-term erythromycin (for 10–14 days) to eradicate B. pertussis from the nasopharynx. Erythromycin and clarithromycin are contraindicated for use in neonates (i.e., <1 month old). Evidence-based medical reports have linked the use of erythromycin to the development of infantile hypertrophic pyloric stenosis (IHPS) in neonates [105, 111]. Azithromycin is recommended by the American Academy of Pediatrics as the antibiotic of choice for neonates (<1 month old) [105]. However, recent research has suggested a strong association between azithromycin exposure and the risk of IHPS in the first 2 weeks of life, with an adjusted odds ratio of 8.26 (95 % confidence interval 2.62–26.0) [112].
Trimethoprim–sulfamethoxazole (TMP–SMX) is used as an alternative to macrolides but should be limited to use in children aged >1 month and in adults when macrolides are not well tolerated [105]. In addition, because of the potential risk of kernicterus in newborns, pregnant women and nursing mothers should avoid ingestions of TMP–SMX [105].
In severe pertussis, exchange transfusion therapy has demonstrated effectiveness when administered early in the disease course. The goal of exchange transfusion is reduction of hyperleukocytosis, which may be as high as 100,000/mm3 [113–121].
6 Pertussis Immunization
Routine childhood immunization is the most effective strategy to prevent transmission and disease associated with pertussis and other vaccine-preventable diseases. Pertussis-containing vaccine schedules, including the primary immunization series, vary considerably from country to country. Although diphtheria–tetanus–acellular pertussis (DTaP) vaccines have become widely used by many developing and developed countries [122–124], a few countries (e.g., India and Poland) offer whole-cell pertussis-containing (DTwP) vaccine in their national childhood immunization schedules [124, 125]. India administers DTwP to children aged 6, 10, and 14 weeks, 16–24 months, and 5 years. In contrast, Germany immunizes children by using DTaP at ages 2, 3, 4, and 11 months [124], while countries such as Italy, Finland, Norway, and Sweden offer DTaP vaccines at 3, 5, and 11–13 months of age. Immunization schedules for pertussis vaccines used in infants and children across the EU can be found online at https://euvac.net and through the ECDC [126]. In the USA, the primary vaccination series for DTaP vaccine includes four doses at the ages of 2, 4, 6, and 15–18 months, with a fifth booster dose at the age of 4–6 years [123, 127].
It is well known that immunity to pertussis is not long-lasting. The results of several epidemiologic studies have estimated that protection following natural infection may last for up to 30 years [128–131]. Other studies have found that protection gained from administration of whole-cell pertussis vaccines may last for up to 14 years [132–138]. However, the immunity following immunization with acellular pertussis vaccines ranges from 4 to 7 years [135, 139–143]. The available data suggest that licensed acellular pertussis vaccines may be associated with faster waning of immunity, which may enable increased pertussis transmission among adults, children, and neonates, in comparison with the available whole-cell vaccines [144].
In the last 10 years, >80 % of pertussis-associated deaths have occurred among neonates and infants under 3 months of age [145]. In response to this observed disease burden, selected countries (i.e., the USA and the UK) have recommended maternal Tdap vaccination [146, 147]. The goal of maternal immunization is to protect young infants, particularly those <3 months of age, through transplacental passive transfer of IgG antibodies to newborns. Maternal pertussis immunization during the third trimester of pregnancy appears to offer optimal antibody transfer to newborns. In general, pertussis-containing vaccines are considered safe for the mother and the infant, as well as resulting in relatively high B. pertussis IgG antibody concentrations [146, 148–150]. However, studies have shown that antibody transfer conferred neither optimal nor long-lasting protection [148, 151–153].
In 2011, the CDC Advisory Committee on Immunization Practice (ACIP) recommended routine Tdap vaccination of all pregnant women after 20 weeks’ gestation. During the ACIP’s deliberations, it was noted that antibody titers decline within 1 year post-Tdap vaccination in healthy adults, including women in the postpartum period [154–157]. For this reason, the ACIP has now recommended that women receive one dose of Tdap in the third trimester of each pregnancy [146, 148]. For women who have missed Tdap vaccination during pregnancy, they may still receive a dose immediately postpartum, with benefit to the mother as well as her newborn baby. Although the rate of Tdap vaccine uptake among mothers increases in the postpartum period, the impact of postpartum Tdap immunization on newborns is less than that of Tdap vaccine administered during pregnancy [158]. In 2012, Castagnini et al. conducted a cross-sectional study to compare the effectiveness of postpartum Tdap immunization among a predominantly Hispanic urban population in Texas before and after release of the 2008 CDC recommendation for postpartum Tdap vaccination. No differences in pertussis hospitalizations, hospital lengths of stay or deaths associated with infant pertussis were observed from the pre- to the post-recommendation eras. The adjusted p value, odds ratio, and 95 % confidence interval were 0.87, 1.06, and 0.5–2.2, respectively [159].
Although Tdap vaccination is strongly recommended for pregnant women in the USA, national reports indicate that Tdap vaccine uptake during pregnancy is low. The California Department of Public Health estimated that only 25 % of pregnant women received the vaccine in 2013 [160]. Other nationwide estimates of Tdap vaccine uptake showed that <3 % of pregnant women are immunized against pertussis during pregnancy [161].
In many countries—including the USA, France, and Australia—a “cocooning” strategy has been implemented to protect young infants who are too young to be immunized or who may be partially immunized [162]. This strategy involves immunization of all household contacts (with DTwP/DTaP or Tdap as appropriate for age), including parents and other family members immediately after childbirth [153, 163]. However, complete immunization of household members and other contacts whom the newborn may encounter poses substantial programmatic challenges to ensure widespread vaccine availability, as well as provider education and outreach to ensure timely Tdap immunization of fathers and postpartum women and routine DTaP immunization of age-eligible children [164, 165]. Encouragingly, recent data reported in 2014 from the UK suggested that maternal Tdap immunization is 91 % effective in preventing pertussis among newborns <8 weeks of age [166].
Many industrialized countries—including the USA, Canada, France, Germany, and Australia—have introduced Tdap vaccine into their adult immunization programs to reduce pertussis-associated morbidity and mortality in young infants [124]. The ACIP recommends a single-dose routine Tdap immunization of adolescents at 11–18 years of age (preferably at ages 11–12 years) regardless of timing from the most recent DTaP dose. For adult age groups, the ACIP recommends a single dose of Tdap in place of tetanus–diphtheria (Td) vaccine for persons aged 19–64 years, and for those aged 65 years and older who have not received Tdap vaccine previously, a single dose of Tdap vaccine is also recommended [167]. Although Tdap vaccines are widely available, the overall uptake of Tdap vaccine among adults remains relatively low (~14 %). In contrast, among adolescents aged 13–17 years, the CDC showed that the uptake of Tdap vaccine was ~80 % in 2012.
Many studies have supported vaccination of healthcare workers (HCWs) because they place patients at increased risk of acquisition of B. pertussis, particularly unimmunized individuals, partially immunized infants, and immunocompromised individuals [67, 168–173]. Despite recommendations for HCWs Tdap immunization, vaccine acceptability among HCWs and Tdap immunization rates are not optimal. In 2012, Walther and colleagues implemented an HCWs immunization program for a Swiss medical institution soon after national adoption of an HCWs Tdap immunization recommendation [174]. Between 2012 and 2013, of 852 eligible HCWs, 427 (51 %) responded to the call. Seventy-two (17 %) had already been vaccinated with Tdap within the previous 10 years, 304 (71 %) received Tdap, and 12 (3 %) declined Tdap immunization. The research team concluded that comprehensive efforts are necessary to mobilize reluctant HCWs and to achieve higher Tdap vaccine uptake.
7 New Pertussis Vaccine Antigens and Adjuvants
The current limitations of existing whole-cell and acellular pertussis-containing vaccines (e.g., the limited duration of protection) have inspired discussion around strategies for improving the available vaccines. Improved pertussis vaccines would induce high levels of protective antibodies, long-term protection, equivalent or higher vaccine efficacy, and lower rates of adverse events following immunization. To date, acellular pertussis-containing vaccines have contained a maximum of five B. pertussis antigens, including PT, FHA, pertactin (Prn), fimbriae serotype 2 (fim 2) and fimbriae serotype 3 (fim 3). A previously studied antigen, adenylate cyclase (AC), is not included in the currently available acellular pertussis vaccines [175–177]. New research is underway to study the safety and immunogenicity of this potential new addition to the pertussis vaccine antigenic armamentarium.
Other antigens offer potential for improving existing pertussis vaccines. Iron regulatory proteins 1–3 (IRP 1–3) have shown promising results as potential vaccine antigens in animal models [178]. To date, no research has been undertaken to study the addition of IRP 1–3 to currently available acellular pertussis vaccines and potential improvements in vaccine safety and immunogenicity in humans. In addition, an autotransporter (BrkA) was tested in preclinical studies but was found not to induce a significant immune response when added to DTaP and DTwP vaccines in a mouse model [179]. It is important to note that the genetic sequence of BrkA is conserved across all B. pertussis isolates tested, including isolates that are Prn deficient and PT deficient. These observations suggest that BrkA may have a capacity for inducing more broadly protective immunity [180].
Pertussis vaccine adjuvants have also been an area of research interest. Current vaccines employ alum (in the form of aluminum gel or aluminum salts) as an adjuvant, which is believed to provide greater stimulation of the T helper (Th)-2 immune response versus the Th-1 response. However, current evidence suggests that a strong Th-1 immune response is required for clearance of B. pertussis [181, 182]. For this reason, new adjuvants, such as those containing CpG oligodeoxynucleotides, have been developed and tested in animal models. CpG motifs are believed to induce significant Th-1 and Th-17 immune responses and yield longer-lasting immunity [183–186]. Additional work to identify new safe and effective antigens and adjuvants for pertussis vaccines represents an important line of research over the next decade.
8 Live-Attenuated Pertussis Vaccines
In 1980, Roberts and colleagues reported the results of work with a B. pertussis aroA mutant strain, which appeared to induce high-level antibodies against B. pertussis in mice immunized by aerosol with the B. pertussis aroA mutant strain [187]. Such vaccine constructs may have utility when applied in a prime-boost immunization strategy in which the aroA mutant strain is administered as the priming vaccine dose. In addition, a live-attenuated BPZE1 pertussis vaccine has been developed that induces both humoral and cell-mediated immunity [182, 188–190]. Additional animal studies have shown that BPZE1 is safe and may protect airways from allergen-driven interleukin (IL)-4, IL-5, and IL-13 [191, 192]. A BPZE1 vaccine has been studied in a phase I, randomized, double-blind clinical trial and was shown to be safe and to induce transient colonization when administered to healthy adults [193]. Additional clinical trials of the BPZE1 pertussis vaccine will open soon for recruitment in Sweden (ClinicalTrials.gov study ID: NCT02453048).
9 Future Development of New-Generation Pertussis Vaccines
At present, whole-cell and acellular pertussis vaccines are used throughout a range of developed and developing countries. Active development of new-generation pertussis vaccines would benefit all countries but would have particular benefit in saving lives among infants and children living in developing countries. In the older model of vaccine introduction, developed country manufacturers traditionally introduced new vaccines into wealthier nations, and these new vaccines then become available in developing countries several years or decades later. In the past 10 years, innovative models of financing for development and introduction of vaccines, such as the Advanced Market Commitment (AMC) have used pneumococcal conjugate vaccine (PCV) and public–private partnerships (e.g., the Meningitis Vaccine Project), which bring together foundations, manufacturers, research organizations, lending organizations, and government agencies, often under the auspices of the Global Alliance for Vaccines and Immunizations (GAVI) [194]. Accelerating development of needed new-generation pertussis vaccines will require active engagement among leading global health organizations, such as the WHO and the CDC, as well as foundations such as the Bill and Melinda Gates Foundation [195].
10 Conclusions
Despite increases in the vaccination coverage achieved for pertussis-containing vaccines in many developed and developing countries, pertussis control remains elusive, with frequent pertussis outbreaks worldwide. These concerning events highlight the need for more effective surveillance and immunization strategies, as well as new-generation vaccines against pertussis. Future strategies to augment pertussis control efforts should include (1) improved surveillance to monitor for pertussis outbreaks, as well as the emergence of new B. pertussis strains (Prn-deficient strains); (2) more widespread and timely access to pertussis diagnostic tests; (3) immunization programmatic evaluations that improve provider education and accessibility of existing Tdap vaccines for adolescents, pregnant women, and other adults; and (4) rigorous evaluation of carefully implemented cocooning strategies.
Since the development of acellular pertussis vaccines in 1980s, little work has been dedicated to the development of new pertussis vaccines that provide high-level and durable protection against pertussis. Although new B. pertussis antigens, adjuvants, and vaccination routes have been explored in animal models, relatively few new pertussis vaccines have entered human clinical trials. Notably, a promising live-attenuated pertussis vaccine is currently in phase I clinical trials. If this experimental vaccine succeeds through all phases of the clinical trials, it may represent an important addition to the childhood immunization schedule and offer an additional tool for control of adolescent and adult pertussis [196]. Finally, enhancing vaccine uptake among pregnant women, adolescents, and adults, including HCWs, may be essential to reducing the burden of pertussis across a range of developed and developing countries globally.
References
Todar K. Bordetella pertussis and whooping cough. 2008–2012. Available from: http://textbookofbacteriology.net/pertussis.html. Accessed 14 Apr 2015 (Todar’s online textbook of bacteriology).
Long S, Edwards K. Bordetella pertussis (pertussis) and other species. Principles and practice of pediatric infectious diseases. New York: Churchill Livingstone; 2003. p. 880–8.
Oakley C. Jules Jean Baptiste Vincent Bordet. 1870–1961. Biogr Mem Fellows R Soc. 1962;8:19–25.
Crowcroft NS, Stein C, Duclos P, Birmingham M. How best to estimate the global burden of pertussis? Lancet Infect Dis. 2003;3(7):413–8.
Centers for Disease Control and Prevention. Pertussis (whooping cough). 2013. Available from: http://www.cdc.gov/pertussis/about/complications.html. Accessed 22 Dec 2014.
Tiwari T, Murphy TV, Moran J, National Immunization Program. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14)):1–16.
Madid A, Cotton M. Pertussis: an update for general practice. S Afr Fam Pract. 2006;48(4):44–6.
Cherry JD, Heininger U. Pertussis and other Bordetella infections. Textbook of pediatric infectious diseases. 7th ed. Philadelphia, PA: W. B. Saunders; 2012 p. 1616–39e11.
Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326–82.
Anderson HR, Bland JM, Peckham CS. Risk factors for asthma up to 16 years of age: evidence from a national cohort study. Chest. 1987;91(6 Suppl):127S–30S.
Keitel WA, Edwards KM. Pertussis in adolescents and adults: time to reimmunize? Semin Respir Infect. 1995;10(1):51–7.
Hewlett EL, Edwards KM. Clinical practice. Pertussis—not just for kids. N Engl J Med. 2005;352(12):1215–22.
Paisley RD, Blaylock J, Hartzell JD. Whooping cough in adults: an update on a reemerging infection. Am J Med. 2012;125(2):141–3.
Cherry JD. Adult pertussis in the pre- and post-vaccine eras: lifelong vaccine-induced immunity? Expert Rev Vaccines. 2014;13(9):1073–80.
European Center for Disease Prevention and Control. Surveillance report—annual epidemiological report—vaccine-preventable diseases. 2014. Available from: http://ecdc.europa.eu/en/publications/Publications/AER-2014-VPD-FINAL.pdf. Accessed 02 Jul 2015.
Centers for Disease Control and Prevention. Vaccines and preventable diseases: Tdap for pregnant women: information for providers. 2014. Available from: http://www.cdc.gov/vaccines/vpd-vac/pertussis/tdap-pregnancy-hcp.htm. Accessed 25 Mar 2015.
World Health Organization. WHO-recommended surveillance standard of pertussis. 2015. Available from: http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis_standards/en/. Accessed 05 Jul 2015.
World Health Organization. Immunization, vaccines and biologicals (pertussis). 2011. Available from: http://www.who.int/immunization/topics/pertussis/en/. Accessed 31 Jan 2015.
Farizo KM, Cochi SL, Zell ER, Brink EW, Wassilak SG, Patriarca PA. Epidemiological features of pertussis in the United States, 1980–1989. Clin Infect Dis. 1992;14(3):708–19.
Centers for Disease Control and Prevention. Pertussis—United States, 1997–2000. MMWR Morb Mortal Wkly Rep. 2002;51(4):73–6.
Black S. Epidemiology of pertussis. Pediatr Infect Dis J. 1997;16(4 Suppl):S85–9.
Edwards KM. Unraveling the challenges of pertussis. Proc Natl Acad Sci USA. 2014;111(2):575–6.
Eshofonie AO, Lin H, Valcin RP, Martin LR, Grunenwald PE. An outbreak of pertussis in rural Texas: an example of the resurgence of the disease in the United States. J Community Health. 2015;40(1):88–91.
Winter K, Glaser C, Watt J, Harriman K. Centers for disease control and prevention. Pertussis epidemic—California, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(48):1129–32.
Centers for Disease Control and Prevention. Final pertussis surveillance report. 2012. Available from: http://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2012.pdf. Accessed 25 Mar 2015.
Centers for Disease Control and Prevention. Final pertussis surveillance report. 2013. Available from: http://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2013.pdf. Accessed 08 Jan 2015.
Centers for Disease Control and Prevention. 2014 provisional pertussis surveillance report. 2015. Available from: http://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2014.pdf. Accessed 13 Apr 2015.
Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-3):1–34.
Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep. 2006;55(RR-17):1–37.
Omer SB, Pan WK, Halsey NA, Stokley S, Moulton LH, Navar AM, et al. Nonmedical exemptions to school immunization requirements: secular trends and association of state policies with pertussis incidence. JAMA. 2006;296(14):1757–63.
Imdad A, Tserenpuntsag B, Blog DS, Halsey NA, Easton DE, Shaw J. Religious exemptions for immunization and risk of pertussis in New York State, 2000–2011. Pediatrics. 2013;132(1):37–43.
Bradford WD, Mandich A. Some state vaccination laws contribute to greater exemption rates and disease outbreaks in the United States. Health Aff. 2015;34(8):1383–90.
Washington State Department of Health. Pertussis summary for Washington State (confirmed and probable cases). 2013. Accessed from: http://www.doh.wa.gov/Portals/1/Documents/Pubs/348-253-PertussisAnnualSummary.pdf. Accessed 16 Jan 2015.
Centers for Disease Control and Prevention. Pertussis epidemic—Washington, 2012. Morb Mortal Wkly Rep (MMWR). 2012;61(28):517–22.
California Department of Public Health. Pertussis Report. 2015. Available from: http://www.cdph.ca.gov/programs/immunize/Documents/Pertussis_Report_1-7-2015.pdf. Accessed 16 Jan 2015.
Michigan Department of Community Health. Whooping cough (pertussis) in Michigan. 2015. Available from: http://www.michigan.gov/mdch/0,4612,7-132-2942_4911_4914-240419–,00.html. Accessed 02 Feb 2015.
Minnesota Department of Health. Infectious Disease Epidemiology, Prevention and Control Division: 2012 annual report. 2012. Available from: http://www.health.state.mn.us/divs/idepc/2012.pdf. Accessed 05 Feb 2015.
Ohio Department of Health. Pertussis. 2013. Available from: http://www.odh.ohio.gov/features/odhfeatures/pertussis.aspx. Acessed 02 Feb 2015.
Ganeshalingham A, Anderson B, McSharry B, Beca J. The New Zealand pertussis experience. Pediatr Crit Care Med. 2014;15(4_Suppl):143–4.
Choe YJ, Kim JW, Park YJ, Jung C, Bae GR. Burden of pertussis is underestimated in South Korea: a result from an active sentinel surveillance system. Jpn J Infect Dis. 2014;67(3):230–2.
Huang H, Zhu T, Gao C, Gao Z, Liu Y, Ding Y, et al. Epidemiological features of pertussis resurgence based on community populations with high vaccination coverage in China. Epidemiol Infect. 2015;143(9):1950–6.
Gabutti G, Rota MC. Pertussis: a review of disease epidemiology worldwide and in Italy. Int J Environ Res Public Health. 2012;9(12):4626–38.
Celentano LP, Massari M, Paramatti D, et al. Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005;24(9):761–5.
Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics. 2005;115(5):1422–7.
National Health and Family Planning Commission of the People’s Republic of China. The statutory reporting of infectious diseases, 2014. 2015. Available from: http://www.nhfpc.gov.cn/jkj/s3578/201502/847c041a3bac4c3e844f17309be0cabd.shtml. Accessed 21 Sept 2015.
The World Bank. Population, total. 2015. Available from: http://data.worldbank.org/indicator/SP.POP.TOTL. Accessed 21 Sept 2015.
Liu Y, Huang HT, Liu P, Zhang Y, Gao ZG, Shu X, et al. Molecular epidemiologic analysis on confirmed pertussis cases. Chin J Public Health. 2011;27(8):987–9.
Luo P, Zhang SM. Advancements of research on immunity to control pertussis. Chin J Public Health. 2010;6(5):651–3.
Fisman DN, Tang P, Hauck T, Richardson S, Drews SJ, Low DE, et al. Pertussis resurgence in Toronto, Canada: a population-based study including test-incidence feedback modeling. BMC Public Health. 2011;11:694.
Riolo MA, King AA, Rohani P. Can vaccine legacy explain the British pertussis resurgence? Vaccine. 2013;31(49):5903–8.
Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation—two sides of the same coin. Epidemiol Infect. 2014;142(4):685–94.
Brinig MM, Cummings CA, Sanden GN, Stefanelli P, Lawrence A, Relman DA. Significant gene order and expression differences in Bordetella pertussis despite limited gene content variation. J Bacteriol. 2006;188(7):2375–82.
Lee GM, Murphy TV, Lett S, Cortese MM, Kretsinger K, Schauer S, et al. Cost effectiveness of pertussis vaccination in adults. Am J Prevent Med. 2007;32(3):186–93.
Mooi FR. Bordetella pertussis and vaccination: the persistence of a genetically monomorphic pathogen. Infect Genet Evol. 2010;10(1):36–49.
Schellekens J, von Konig CH, Gardner P. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatr Infect Dis J. 2005;24(5 Suppl):S19–24.
Schmidtke AJ, Boney KO, Martin SW, Skoff TH, Tondella ML, Tatti KM. Population diversity among Bordetella pertussis isolates, United States, 1935–2009. Emerg Infect Dis. 2012;18(8):1248–55.
King AJ, van Gorkom T, van der Heide HG, Advani A, van der Lee S. Changes in the genomic content of circulating Bordetella pertussis strains isolated from the Netherlands, Sweden, Japan and Australia: adaptive evolution or drift? BMC Genomics. 2010;11:64.
Forsyth KD, Campins-Marti M, Caro J, Cherry JD, Greenberg D, Guiso N, et al. New pertussis vaccination strategies beyond infancy: recommendations by the Global Pertussis Initiative. Clin Infect Dis. 2004;39(12):1802–9.
Lee LH, Pichichero ME. Costs of illness due to Bordetella pertussis in families. Arch Fam Med. 2000;9(10):989–96.
Lee GM, Lett S, Schauer S, LeBaron C, Murphy TV, Rusinak D, et al. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis. 2004;39(11):1572–80.
Lopez MA, Cruz AT, Kowalkowski MA, Raphael JL. Trends in hospitalizations and resource utilization for pediatric pertussis. Hosp Pediatr. 2014;4(5):269–75.
Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463–70.
Thampi N, Gurol-Urganci I, Crowcroft NS, Sander B. Pertussis post-exposure prophylaxis among household contacts: a cost-utility analysis. PloS one. 2015;10(3):e0119271.
McGarry LJ, Krishnarajah G, Hill G, Masseria C, Skornicki M, Pruttivarasin N, et al. Cost-effectiveness of Tdap vaccination of adults aged ≥65 years in the prevention of pertussis in the US: a dynamic model of disease transmission. PloS One. 2014;9(1):e72723.
Khetsuriani N, Bisgard K, Prevots DR, Brennan M, Wharton M, Pandya S, et al. Pertussis outbreak in an elementary school with high vaccination coverage. Pediatr Infect Dis J. 2001;20(12):1108–12.
Maltezou HC, Ftika L, Theodoridou M. Nosocomial pertussis in neonatal units. J Hosp Infect. 2013;85(4):243–8.
Kuncio DE, Middleton M, Cooney MG, Ramos M, Coffin SE, Feemster KA. Health care worker exposures to pertussis: missed opportunities for prevention. Pediatrics. 2014;133(1):15–21.
Cornia PB, Hersh AL, Lipsky BA, Newman TB, Gonzales R. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304(8):890–6.
Loeffelholz M. Towards improved accuracy of Bordetella pertussis nucleic acid amplification tests. J Clin Microbiol. 2012;50(7):2186–90.
Koidl C, Bozic M, Burmeister A, Hess M, Marth E, Kessler HH. Detection and differentiation of Bordetella spp. by real-time PCR. J Clin Microbiol. 2007;45(2):347–50.
Qin X, Galanakis E, Martin ET, Englund JA. Multitarget PCR for diagnosis of pertussis and its clinical implications. J Clin Microbiol. 2007;45(2):506–11.
Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. Chapter 10: Pertussis, Centers for Disease Control and Prevention, Atlanta, GA, 2008.
Leber AL. Pertussis: relevant species and diagnostic update. Clin Lab Med. 2014;34(2):237–55.
Fry NK, Duncan J, Wagner K, Tzivra O, Doshi N, Litt DJ, et al. Role of PCR in the diagnosis of pertussis infection in infants: 5 years’ experience of provision of a same-day real-time PCR service in England and Wales from 2002 to 2007. J Med Microbiol. 2009;58(Pt 8):1023–9.
The Commonwealth of Massachusetts, Executive Office of Health and Human Services, Department of Public Health, Division of Epidemiology and Immunization. Clinical advisory. 2013. Available from: http://www.mass.gov/eohhs/docs/dph/cdc/immunization/advisory-pertussis-20130815.pdf. Accessed 16 Mar 2015.
Taranger J, Trollfors B, Lind L, Zackrisson G, Beling-Holmquist K. Environmental contamination leading to false-positive polymerase chain reaction for pertussis. Pediatr Infect Dis J. 1994;13(10):936–7.
van der Zee A, Agterberg C, van Agterveld M, Peeters M, Mooi FR. Characterization of IS1001, an insertion sequence element of Bordetella parapertussis. J Bacteriol. 1993;175(1):141–7.
Sloan LM, Hopkins MK, Mitchell PS, Vetter EA, Rosenblatt JE, Harmsen WS, et al. Multiplex LightCycler PCR assay for detection and differentiation of Bordetella pertussis and Bordetella parapertussis in nasopharyngeal specimens. J Clin Microbiol. 2002;40(1):96–100.
van der Zee A, Agterberg C, Peeters M, Mooi F, Schellekens J. A clinical validation of Bordetella pertussis and Bordetella parapertussis polymerase chain reaction: comparison with culture and serology using samples from patients with suspected whooping cough from a highly immunized population. J Infect Dis. 1996;174(1):89–96.
Reischl U, Lehn N, Sanden GN, Loeffelholz MJ. Real-time PCR assay targeting IS481 of Bordetella pertussis and molecular basis for detecting Bordetella holmesii. J Clin Microbiol. 2001;39(5):1963–6.
Tatti KM, Sparks KN, Boney KO, Tondella ML. Novel multitarget real-time PCR assay for rapid detection of Bordetella species in clinical specimens. J Clin Microbiol. 2011;49(12):4059–66.
Tatti KM, Wu KH, Tondella ML, Cassiday PK, Cortese MM, Wilkins PP, et al. Development and evaluation of dual-target real-time polymerase chain reaction assays to detect Bordetella spp. Diagn Microbiol Infect Dis. 2008;61(3):264–72.
Tizolova A, Guiso N, Guillot S. Insertion sequences shared by Bordetella species and implications for the biological diagnosis of pertussis syndrome. Eur J Clin Microbiol Infect Dis. 2013;32(1):89–96.
Jerris RC, Williams SR, MacDonald HJ, Ingebrigtsen DR, Westblade LF, Rogers BB. Testing implications of varying targets for Bordetella pertussis: comparison of the FilmArray Respiratory Panel and the Focus B pertussis PCR assay. J Clin Pathol. 2015;68(5):394–6.
Kamachi K, Toyoizumi-Ajisaka H, Toda K, Soeung SC, Sarath S, Nareth Y, et al. Development and evaluation of a loop-mediated isothermal amplification method for rapid diagnosis of Bordetella pertussis infection. J Clin Microbiol. 2006;44(5):1899–902.
Nakamura A, Sakano T, Nakayama T, Shimoda H, Okada Y, Hanayama R, et al. Neonatal pertussis presenting as acute bronchiolitis: direct detection of the Bordetella pertussis genome using loop-mediated isothermal amplification. Eur J Pediatr. 2009;168(3):347–9.
Torkaman MR, Kamachi K, Nikbin VS, Lotfi MN, Shahcheraghi F. Comparison of loop-mediated isothermal amplification and real-time PCR for detecting Bordetella pertussis. J Med Microbiol. 2015;64(Pt 4):463–5.
Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289(1):150–4.
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63.
Centers for Disease Control and Prevention. Pertussis (whooping cough). 2013. Available from: http://www.cdc.gov/pertussis/clinical/diagnostic-testing/specimen-collection.html. Accessed 31 Jan 2015.
Leber AL, Salamon DP, Prince HE. Pertussis diagnosis in the 21st century: progress and pitfalls, part II. Clin Microbiol Newsl. 2011;33(16):119–26.
Kline JM, Lewis WD, Smith EA, Tracy LR, Moerschel SK. Pertussis: a reemerging infection. Am Fam Physician. 2013;88(8):507–14.
World Health Organization. WHO-recommended standards for surveillance of selected vaccine-preventable diseases. 2003. Available from: http://whqlibdoc.who.int/hq/2003/who_v&b_03.01.pdf. Accessed 16 Mar 2015.
Marchant CD, Loughlin AM, Lett SM, Todd CW, Wetterlow LH, Bicchieri R, et al. Pertussis in Massachusetts, 1981–1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis. 1994;169(6):1297–305.
Muller FM, Hoppe JE, Wirsing von Konig CH. Laboratory diagnosis of pertussis: state of the art in 1997. J Clin Microbiol. 1997;35(10):2435–43.
Cherry JD, Grimprel E, Guiso N, Heininger U, Mertsola J. Defining pertussis epidemiology: clinical, microbiologic and serologic perspectives. Pediatr Infect Dis J. 2005;24(5 Suppl):S25–34.
Isacson J, Trollfors B, Hedvall G, Taranger J, Zackrisson G. Response and decline of serum IgG antibodies to pertussis toxin, filamentous hemagglutinin and pertactin in children with pertussis. Scand J Infect Dis. 1995;27(3):273–7.
Hallander HO. Microbiological and serological diagnosis of pertussis. Clin Infect Dis. 1999;28(Suppl 2):S99–106.
Greenberg DP. Pertussis in adolescents: increasing incidence brings attention to the need for booster immunization of adolescents. Pediatr Infect Dis J. 2005;24(8):721–8.
Pawloski LC, Kirkland KB, Baughman AL, Martin MD, Talbot EA, Messonnier NE, et al. Does tetanus–diphtheria–acellular pertussis vaccination interfere with serodiagnosis of pertussis infection? Clin Vaccine Immunol CVI. 2012;19(6):875–80.
Berti E, Venturini E, Galli L, de Martino M, Chiappini E. Management and prevention of pertussis infection in neonates. Expert Rev Anti Infect Ther. 2014;12(12):1515–31.
Edwards KM, Decker MD. Pertussis vaccines, vaccines. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013. p. 447–92.
Centers for Disease Control and Prevention. Pertussis (whooping cough): guidelines for the control of pertussis outbreaks. 2013. Available from: http://www.cdc.gov/pertussis/outbreaks/guide/index.html. Accessed 15 Feb 2015.
Murphy TV, Slade BA, Broder KR, Kretsinger K, Tiwari T, Joyce PM, et al. Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-4):1–51.
Tiwari T, Murphy TV, Moran J. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis. MMWR Recomm Rep. 2005;54:1–16.
Bettiol S, Wang K, Thompson MJ, Roberts NW, Perera R, Heneghan CJ, et al. Symptomatic treatment of the cough in whooping cough. Cochrane database Syst Rev. 2012;5:CD003257.
Altunaiji S, Kukuruzovic R, Curtis N, Massie J. Antibiotics for whooping cough (pertussis). Cochrane database Syst Rev. 2007(3):CD004404.
Langley JM, Halperin SA, Boucher FD, Smith B. Pediatric Investigators Collaborative Network on Infections in Canada. Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics. 2004;114(1):e96–101.
Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev. 2008;9(3):201–12. doi:10.1016/j.prrv.2008.05.010.
Halperin SA, Bortolussi R, Langley JM, Eastwood BJ, De Serres G. A randomized, placebo-controlled trial of erythromycin estolate chemoprophylaxis for household contacts of children with culture-positive Bordetella pertussis infection. Pediatrics. 1999;104(4):e42.
Lozada LE, Royall MJ, Nylund CM, Eberly MD. Development of pyloric stenosis after a 4-day course of oral erythromycin. Pediatr Emerg Care. 2013;29(4):498–9.
Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics. 2015;135(3):483–8.
Nieves D, Bradley JS, Gargas J, Mason WH, Lehman D, Lehman SM, et al. Exchange blood transfusion in the management of severe pertussis in young infants. Pediatr Infect Dis J. 2013;32(6):698–9.
Romano MJ, Weber MD, Weisse ME, Siu BL. Pertussis pneumonia, hypoxemia, hyperleukocytosis, and pulmonary hypertension: improvement in oxygenation after a double volume exchange transfusion. Pediatrics. 2004;114(2):e264–6.
Donoso AF, Cruces PI, Camacho JF, Leon JA, Kong JA. Exchange transfusion to reverse severe pertussis-induced cardiogenic shock. Pediatr Infect Dis J. 2006;25(9):846–8.
Grzeszczak MJ, Churchwell KB, Edwards KM, Pietsch J. Leukopheresis therapy for severe infantile pertussis with myocardial and pulmonary failure. Pediatr Crit Care Med. 2006;7(6):580–2.
Martinez M, Rochat I, Corbelli R, Tissieres P, Rimensberger PC, Barazzone-Argiroffo C. Early blood exchange transfusion in malignant pertussis: a case report. Pediatr Crit Care Med. 2011;12(2):e107–9.
Rowlands HE, Goldman AP, Harrington K, Karimova A, Brierley J, Cross N, et al. Impact of rapid leukodepletion on the outcome of severe clinical pertussis in young infants. Pediatrics. 2010;126(4):e816–27.
Tzeng M, Petroski R, Gharpure V. 1266: succesful use of exchange transfusion for malignant pertussis. Crit Care Med. 2013;41(12):A325–6.
Kuperman A, Hoffmann Y, Glikman D, Dabbah H, Zonis Z. Severe pertussis and hyperleukocytosis: is it time to change for exchange? Transfusion. 2014;54(6):1630–3.
Chantreuil J, Fakhri N, Labarthe F, Saliba E, Favrais G. Malignant pertussis and exchange transfusion. Arch Pediatr. 2015;22(1):84–7.
Kuno-Sakai H, Kimura M. Safety and efficacy of acellular pertussis vaccine in Japan, evaluated by 23 years of its use for routine immunization. Pediatr Int. 2004;46(6):650–5.
ACIP Childhood/Adolescent Immunization Work Group, Akinsanya-Beysolow I, Jenkins R, Meissner HC, Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for persons aged 0 through 18 years—United States. MMWR Surveill Summ. 2013;62(Suppl 1):2–8.
World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2015 global summary. 2015. Available from: http://apps.who.int/immunization_monitoring/globalsummary/schedules. Accessed 06 Jul 2015.
Indian Academy of Pediatrics. IAP recommended immunization schedule 2013 for children aged 0–18 years (with range). 2013. Available from: http://www.iapindia.org/IMM%20Schedule.pdf. Accessed 18 Dec 2014.
European Center for Disease Prevention and Control. Scientific panel on childhood immunisation schedule: diphtheria–tetanus–pertussis (DTP) vaccination. 2009. Available from: http://ecdc.europa.eu/en/publications/Publications/0911_GUI_Scientific_Panel_on_Childhood_Immunisation_DTP.pdf. Accessed 14 Sept 2015.
Brady MT, Byington CL, Davies HD, Edwards KM, Jackson MA, Maldonado YA, et al. Recommended childhood and adolescent immunization schedule—United States, 2014. Pediatrics. 2014;133(2):357–63.
Wirsing von Konig CH, Postels-Multani S, Bock HL, Schmitt HJ, Pertussis in adults: frequency of transmission after household exposure. Lancet. 1995;346(8986):1326–9.
Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24(5 Suppl):S58–61.
Versteegh FG, Schellekens JF, Nagelkerke AF, Roord JJ. Laboratory-confirmed reinfections with Bordetella pertussis. Acta Paediatr. 2002;91(1):95–7.
Wearing HJ, Rohani P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. 2009;5(10):e1000647.
Grimprel E, Begue P, Anjak I, Njamkepo E, Francois P, Guiso N. Long-term human serum antibody responses after immunization with whole-cell pertussis vaccine in France. Clin Diagn Lab Immunol. 1996;3(1):93–7.
He Q, Viljanen MK, Nikkari S, Lyytikainen R, Mertsola J. Outcomes of Bordetella pertussis infection in different age groups of an immunized population. J Infect Dis. 1994;170(4):873–7.
Jenkinson D. Duration of effectiveness of pertussis vaccine: evidence from a 10 year community study. Br Med J (Clin Res Ed). 1988;296(6622):612–4.
Lugauer S, Heininger U, Cherry JD, Stehr K. Long-term clinical effectiveness of an acellular pertussis component vaccine and a whole cell pertussis component vaccine. Eur J Pediatr. 2002;161(3):142–6.
Torvaldsen S, Simpson JM, McIntyre PB. Effectiveness of pertussis vaccination in New South Wales, Australia, 1996–1998. Eur J Epidemiol. 2003;18(1):63–9.
He Q, Schmidt-Schlapfer G, Just M, Matter HC, Nikkari S, Viljanen MK, et al. Impact of polymerase chain reaction on clinical pertussis research: Finnish and Swiss experiences. J Infect Dis. 1996;174(6):1288–95.
Van Buynder PG, Owen D, Vurdien JE, Andrews NJ, Matthews RC, Miller E. Bordetella pertussis surveillance in England and Wales: 1995–7. Epidemiol Infect. 1999;123(3):403–11.
Salmaso S, Mastrantonio P, Tozzi AE, Stefanelli P, Anemona A, Ciofi degli Atti ML, et al. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. Pediatrics. 2001;108(5):E81.
Guiso N, Begue P, Cohen R. Comparison of pertussis antibody levels in children up to 5 years of age primed at 2, 3, 4 months and boostered in a second year of life with either DTPa or DTPW based combination vaccines in France. Toronto; Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000.
Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–9.
Tindberg Y, Blennow M, Granstrom M. A ten year follow-up after immunization with a two component acellular pertussis vaccine. Pediatr Infect Dis J. 1999;18(4):361–5.
Gustafsson L, Hessel L, Storsaeter J, Olin P. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5, and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics. 2006;118(3):978–84.
World Health Organization. Weekly epidemiological record—revised guidance on the choice of pertussis vaccines: July 2014. 2014. Available from: http://www.who.int/wer/2013/wer8930.pdf?ua=1. Accessed 07 July 2015.
Centers for Disease Control and Prevention. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington D.C: Public Health Foundation; 2015.
Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morbid Mortal Wkly Rep. 2011;60(41):1424.
Joint Committee on Vaccination and Immunization. Minutes of teleconference on Thursday 30 August 2012 10.00am—12.00am and post-teleconference discussion. 2012. Available from: www.gov.uk/government/uploads/system/uploads/attachment_data/file/223497/JCVI_minutes_Aug_2012_Pertussis_-_final.pdf. Accessed 14 Sept 2015.
Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morbid Mortal Wkly Rep. 2013;62(7):131.
Curran D. Tdap vaccination in pregnancy: new guidance, new challenges. N C Med J. 2012;74(5):421–2.
Halperin BA, Morris A, Mackinnon-Cameron D, Mutch J, Langley JM, McNeil SA, et al. Kinetics of the antibody response to tetanus–diphtheria–acellular pertussis vaccine in women of childbearing age and postpartum women. Clin Infect Dis. 2011;53(9):885–92.
Shakib JH, Ralston S, Raissy HH, Stoddard GJ, Edwards KM, Byington CL. Pertussis antibodies in postpartum women and their newborns. J Perinatol. 2010;30(2):93–7.
Hardy-Fairbanks AJ, Pan SJ, Decker MD, Johnson DR, Greenberg DP, Kirkland KB, et al. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. Pediatr Infect Dis J. 2013;32(11):1257–60.
Locht C, Mielcarek N. New pertussis vaccination approaches: en route to protect newborns? FEMS Immunol Med Microbiol. 2012;66(2):121–33.
Weston W, Messier M, Friedland LR, Wu X, Howe B. Persistence of antibodies 3 years after booster vaccination of adults with combined acellular pertussis, diphtheria and tetanus toxoids vaccine. Vaccine. 2011;29(47):8483–6.
Booy R, Van der Meeren O, Ng SP, Celzo F, Ramakrishnan G, Jacquet JM. A decennial booster dose of reduced antigen content diphtheria, tetanus, acellular pertussis vaccine (Boostrix) is immunogenic and well tolerated in adults. Vaccine. 2010;29(1):45–50.
Tomovici A, Barreto L, Zickler P, Meekison W, Noya F, Voloshen T, et al. Humoral immunity 10 years after booster immunization with an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine. Vaccine. 2012;30(16):2647–53.
Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013;56(4):539–44.
Liang JL. “Cocooning” and Tdap vaccination. 2015. Available from: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/pertussis-02-liang.pdf. Accessed 14 Sept 2015.
Castagnini LA, Healy CM, Rench MA, Wootton SH, Munoz FM, Baker CJ. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis. 2012;54(1):78–84.
Harriman K, Winter K. Pertussis vaccine uptake during pregnancy: we need to do better in the US. Prev Med. 2014;67:320–1.
Liang J. Considerations for recommendation on Tdap for every pregnancy. Advisory Committee on Immunization Practices (ACIP), Atlanta; 2012.
World Health Organization. Pertussis vaccines: WHO position paper. Weekly Epidemiological Record. 2010. Available from: www.who.int/wer/2010/wer8540.pdf. Accessed 06 Jul 2015.
de Greeff SC, de Melker HE, Westerhof A, Schellekens JF, Mooi FR, van Boven M. Estimation of household transmission rates of pertussis and the effect of cocooning vaccination strategies on infant pertussis. Epidemiology. 2012;23(6):852–60.
Skowronski DM, Janjua NZ, Tsafack EP, Ouakki M, Hoang L, De Serres G. The number needed to vaccinate to prevent infant pertussis hospitalization and death through parent cocoon immunization. Clin Infect Dis. 2012;54(3):318–27.
Munoz F, Englund J. Infant pertussis: is cocooning the answer? Clin Infect Dis. 2011;53(9):893–6.
Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, et al. A case–control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis. 2015;60(3):333–7.
Bridges CB, Woods ML, Coyne-Beasley T. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older—United States, 2013. MMWR Surveill Summ. 2013;62(Suppl 1):9–19.
Campins-Martı M, Cheng H, Forsyth K, Guiso N, Halperin S, Huang L-M, et al. Recommendations are needed for adolescent and adult pertussis immunisation: rationale and strategies for consideration. Vaccine. 2001;20(5):641–6.
Schellekens J, von König C-HW, Gardner P. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatr Infect Dis J. 2005;24(5):S19–24.
Bechini A, Tiscione E, Boccalini S, Levi M, Bonanni P. Acellular pertussis vaccine use in risk groups (adolescents, pregnant women, newborns and health care workers): a review of evidences and recommendations. Vaccine. 2012;30(35):5179–90.
Shefer A, Strikas R, Bridges CB. Updated recommendations of the Advisory Committee on Immunization Practices for healthcare personnel vaccination: a necessary foundation for the essential work that remains to build successful programs. Infect Control. 2012;33(01):71–4.
Maltezou HC, Katerelos P, Poufta S, Pavli A, Maragos A, Theodoridou M. Attitudes toward mandatory occupational vaccinations and vaccination coverage against vaccine-preventable diseases of health care workers in primary health care centers. Am J Infect Control. 2013;41(1):66–70.
Heininger U. Vaccination of health care workers against pertussis: meeting the need for safety within hospitals. Vaccine. 2014;32(38):4840–3.
Walther K, Burckhardt MA, Erb T, Heininger U. Implementation of pertussis immunization in health-care personnel. Vaccine. 2015;33(17):2009–14.
Guiso N, Szatanik M, Rocancourt M. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microbiol Pathog. 1991;11(6):423–31.
Macdonald-Fyall J, Xing D, Corbel M, Baillie S, Parton R, Coote J. Adjuvanticity of native and detoxified adenylate cyclase toxin of Bordetella pertussis towards co-administered antigens. Vaccine. 2004;22(31–32):4270–81.
Cheung GY, Xing D, Prior S, Corbel MJ, Parton R, Coote JG. Effect of different forms of adenylate cyclase toxin of Bordetella pertussis on protection afforded by an acellular pertussis vaccine in a murine model. Infect Immun. 2006;74(12):6797–805.
Alvarez Hayes J, Erben E, Lamberti Y, Ayala M, Maschi F, Carbone C, et al. Identification of a new protective antigen of Bordetella pertussis. Vaccine. 2011;29(47):8731–9.
Marr N, Oliver DC, Laurent V, Poolman J, Denoel P, Fernandez RC. Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model. Vaccine. 2008;26(34):4306–11.
Bouchez V, Brun D, Cantinelli T, Dore G, Njamkepo E, Guiso N. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine. 2009;27(43):6034–41.
Mills KH, Ryan M, Ryan E, Mahon BP. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun. 1998;66(2):594–602.
Feunou PF, Bertout J, Locht C. T- and B-cell-mediated protection induced by novel, live attenuated pertussis vaccine in mice: cross protection against parapertussis. PloS One. 2010;5(4):e10178.
Sugai T, Mori M, Nakazawa M, Ichino M, Naruto T, Kobayashi N, et al. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria–tetanus–pertussis vaccine. Vaccine. 2005;23(46–47):5450–6.
Polewicz M, Gracia A, Buchanan R, Strom S, Halperin SA, Potter AA, et al. Influence of maternal antibodies on active pertussis toxoid immunization of neonatal mice and piglets. Vaccine. 2011;29(44):7718–26.
Gracia A, Polewicz M, Halperin SA, Hancock RE, Potter AA, Babiuk LA, et al. Antibody responses in adult and neonatal BALB/c mice to immunization with novel Bordetella pertussis vaccine formulations. Vaccine. 2011;29(8):1595–604.
Garlapati S, Eng NF, Kiros TG, Kindrachuk J, Mutwiri GK, Hancock RE, et al. Immunization with PCEP microparticles containing pertussis toxoid, CpG ODN and a synthetic innate defense regulator peptide induces protective immunity against pertussis. Vaccine. 2011;29(38):6540–8.
Roberts M, Maskell D, Novotny P, Dougan G. Construction and characterization in vivo of Bordetella pertussis aroA mutants. Infect Immun. 1990;58(3):732–9.
Mielcarek N, Debrie AS, Raze D, Bertout J, Rouanet C, Younes AB, et al. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2006;2(7):e65.
Feunou PF, Kammoun H, Debrie AS, Mielcarek N, Locht C. Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine. 2010;28(43):7047–53.
Mielcarek N, Debrie AS, Mahieux S, Locht C. Dose response of attenuated Bordetella pertussis BPZE1-induced protection in mice. Clin Vaccine Immunol. 2010;17(3):317–24.
Kavanagh H, Noone C, Cahill E, English K, Locht C, Mahon BP. Attenuated Bordetella pertussis vaccine strain BPZE1 modulates allergen-induced immunity and prevents allergic pulmonary pathology in a murine model. Clin Exp Allergy. 2010;40(6):933–41.
Li R, Lim A, Phoon MC, Narasaraju T, Ng JK, Poh WP, et al. Attenuated Bordetella pertussis protects against highly pathogenic influenza A viruses by dampening the cytokine storm. J Virol. 2010;84(14):7105–13.
Thorstensson R, Trollfors B, Al-Tawil N, Jahnmatz M, Bergstrom J, Ljungman M, et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine—BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PloS One. 2014;9(1):e83449.
Snyder CM, Begor W, Berndt ER. Economic perspectives on the advance market commitment for pneumococcal vaccines. Health affairs. 2011;30(8):1508–17.
Grace C. Developing new technologies to address neglected diseases: the role of product development partnerships and advanced market commitments. Department for International Development (DFID) Health Research Center Report; 2006.
Locht C. A common vaccination strategy to solve unsolved problems of tuberculosis and pertussis? Microbes Infect Inst Pasteur. 2008;10(9):1051–6.
Loeffelholz MJ, Thompson CJ, Long KS, Gilchrist MJ. Comparison of PCR, culture, and direct fluorescent-antibody testing for detection of Bordetella pertussis. J Clin Microbiol. 1999;37(9):2872–6.
Halperin SA, Bortolussi R, Wort AJ. Evaluation of culture, immunofluorescence, and serology for the diagnosis of pertussis. J Clin Microbiol. 1989;27(4):752–7.
Lind-Brandberg L, Welinder-Olsson C, Lagergard T, Taranger J, Trollfors B, Zackrisson G. Evaluation of PCR for diagnosis of Bordetella pertussis and Bordetella parapertussis infections. J Clin Microbiol. 1998;36(3):679–83.
Patriarca PA, Biellik RJ, Sanden G, Burstyn DG, Mitchell PD, Silverman PR, et al. Sensitivity and specificity of clinical case definitions for pertussis. Am J Public Health. 1988;78(7):833–6.
Centers for Disease Control and Prevention. Pertussis (whooping cough). 2012. Available from: http://www.cdc.gov/pertussis/clinical/diagnostic-testing/diagnosis-pcr-bestpractices.html. Accessed 18 Mar 2015.
Andre P, Caro V, Njamkepo E, Wendelboe AM, Van Rie A, Guiso N. Comparison of serological and real-time PCR assays to diagnose Bordetella pertussis infection in 2007. J Clin Microbiol. 2008;46(5):1672–7.
de Melker HE, Versteegh FG, Conyn-Van Spaendonck MA, Elvers LH, Berbers GA, van Der Zee A, et al. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J Clin Microbiol. 2000;38(2):800–6.
Yih WK, Lett SM, des Vignes FN, Garrison KM, Sipe PL, Marchant CD. The increasing incidence of pertussis in Massachusetts adolescents and adults, 1989–1998. J Infect Dis. 2000;182(5):1409–16.
von Konig CHW, Gounis D, Laukamp S, Bogaerts H, Schmitt HJ. Evaluation of a single-sample serological technique for diagnosing pertussis in unvaccinated children. Eur J Clin Microbiol Infect Dis. 1999;18(5):341–5.
Halperin SA, Bortolussi R, Langley JM, Miller B, Eastwood BJ. Seven days of erythromycin estolate is as effective as fourteen days for the treatment of Bordetella pertussis infections. Pediatrics. 1997;100(1):65–71.
Gregory DS. Pertussis: a disease affecting all ages. Am Fam Physician. 2006;74(3):420–6.
Acknowledgments
All authors—Abdulbaset Salim, Yan Liang, and Paul Kilgore—played a key role in the development of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this work.
Conflict of interest
Abdulbaset Salim, Yan Liang, and Paul Kilgore declare that there are no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Salim, A.M., Liang, Y. & Kilgore, P.E. Protecting Newborns Against Pertussis: Treatment and Prevention Strategies. Pediatr Drugs 17, 425–441 (2015). https://doi.org/10.1007/s40272-015-0149-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-015-0149-x