Abstract
We present the case of a rare entity which is a complication of a disease process that had almost disappeared from the Western World. With the recent resurgence in reported cases of Mycobacterium tuberculosis (TB) in Western communities, it is important to recognize complications and sequelae. A young alcoholic male with confirmed active TB suffered a cardiac arrest following massive haemoptysis. Multidetector computed tomography angiography diagnosed a Rasmussen’s aneurysm, confirmed by digital subtraction angiography and then successfully embolized with glue. We outline this rare case and the embolization technique and review previously documented reports.
Similar content being viewed by others
Tuberculosis (TB) was the dreaded disease of the early 20th century, with many lives lost and debilitated by this bacterium. Following the introduction of antituberculous chemotherapy in the 1940s, some control was gained over this plague. However, there is now a resurgence in reported cases of Mycobacterium tuberculosis in Western communities, particularly among intravenous drug abusers and alcoholics. Pulmonary manifestations are the most common sequelae of this inhaled bacterium, comprising lesions of the lung parenchyma, airways, vasculature, mediastinum, pleura, and chest wall [1]. All of these manifestations and complications can be readily diagnosed radiologically and many can also be treated by interventional radiologists.
Hemoptysis in the presence of TB, often massive, can result from a number of different etiologies, namely, bronchiectasis, aspergilloma, TB reactivation, scar carcinoma, chronic bronchitis, microbial colonization within a cavity, and vascular complications such as pseudoaneurysms [2]. Minor hemoptysis is usually self- limiting or controlled with anti-TB chemotherapy, however, massive, life-threatening hemoptysis is increasingly being managed with arterial embolization [3]. Usually, the source of bleeding is the bronchial arteries due to the surrounding chronic lung parenchymal inflammation, resulting in hypervascularity and elevated pressure within the bronchial arteries or arteriovenous fistula formation [4, 5]. Another, rarer etiology of hemoptysis in TB is due to pulmonary arterial bleeding from a Rasmussen’s aneurysm. Despite Rasmussen’s aneurysms being reported in up to 5% of autopsy series of patients with cavitary lesions [6], relatively few case reports exist in the literature [1, 6–14]. We report an unusual case of a young male alcoholic with active cavitary TB who suffered a cardiac arrest following massive hemoptysis. Multidetector computed tomography angiography (MDCTA) diagnosed a Rasmussen’s aneurysm, confirmed by digital subtraction angiography and then successfully embolized with NBCA-MS co-monomer (Glubran 2; GEM, Viareggio, Italy). We outline this rare case and the embolization technique and review the literature on this condition.
Case Report
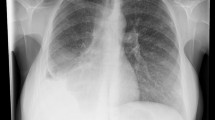
A 40-year-old male alcoholic presented to a regional hospital with a 3-month history of cough, weight loss, and drenching night sweats. Delay in seeking medical attention was due to the patient’s alcoholism. Initial plain chest x-ray diagnosed multiple cavities throughout both lungs, with the largest in the left base (Fig. 1). Sputum was positive for acid-fast bacilli and a diagnosis of active pulmonary TB was made. Admission to hospital and isolation were required to actively manage this patient due to his wayward lifestyle. Triple antituberculous chemotherapy was commenced, with rifampicin, isoniazid, and pyrazinamide orally on a supervised basis. After 1 week in the hospital, the patient had a cardiac arrest following a massive hemoptysis with an episode of asystole followed by pulseless electrical activity. Hemoglobin dropped precipitously, from 12 to 6.4 g/dl. Fluid and blood resuscitation stabilized the patient’s blood pressure, and subsequent intubation and ventilation secured his airway. A chest radiograph demonstrated left lower lobe collapse. A contrast-enhanced 64-slice MDCT angiogram was performed and demonstrated numerous lung parenchymal cavities, one of which was associated with an aneurysm of a branch of the left lower lobe pulmonary artery (Fig. 2). At this point the patient was transferred to our institution to undergo embolization of this Rasmussen’s aneurysm.
Embolization Procedure
While the patient was intubated and ventilated, his right femoral vein was cannulated and a 6-Fr sheath (Avanti; Cordis, Roden, The Netherlands) inserted. A regular J guidewire (standard wire guide, 145 cm, 0.035 in.; Cook; William Cook Europe, Bjaeverskov, Denmark) followed by a Pigtail catheter (Royal Flush Plus Pigtail Angiographic Beacon Tip Catheter; Cook, Bjaeverskov, Denmark) was inserted into the left main pulmonary artery. digital subtraction angiography confirmed the presence of the Rasmussen’s aneurysm arising from a lower lobe pulmonary arterial branch (Fig. 3). Selective angiography with a Hockey stick catheter (Cook) confirmed a narrow neck configuration. A microcatheter (Progreat; 2.4–2.8 Fr; Terumo; Leuven, Belgium) and microwire (Progreat; 0.018 in.; Terumo) were employed to enter the aneurysm sac (Fig. 4) and for embolization material deployment, to ensure accurate and site-specific delivery. Glue was elected as the embolization material of choice for this case. A Glubran:Lipiodol (Glubran 2, NBCA–MS co-monomer [GEM]; Lipiodol, 480 mg I/ml [Guerbet, Roissy Ch de Gaulle, France]) mixture at the ratio of 1:2 was used to embolize this vascular abnormality. The aneurysm itself was successfully occluded with Glubran 2, as demonstrated by the postembolization angiogram (Fig. 5). There was no migration of Glubran 2 into any of the surrounding normal arteries.
The patient remained sedated and intubated for 3 days following the embolization to ensure no forced coughing. There were no further episodes of hemoptysis, and the hemoglobin remained stable at 10 g/dl following the initial blood transfusion. Unfortunately, it was difficult to wean the patient from the ventilator and he was diagnosed with anoxic brain injury, as a result of the initial cardiac arrest.
Discussion
Transcatheter embolization used to be a “last resort” therapy, when surgical techniques had failed or were considered unfeasible, however, now emergency arterial embolization is becoming a standard first-line treatment option for the management of unstable patients with acute arterial bleeding from all sources. Remy et al. reported that pulmonary arterial embolization was safe, logical, and successful in pulmonary arteriovenous malformations and hemoptysis of pulmonary arterial origin [8, 15]. There are few other large series available detailing pulmonary arterial embolization, as the bronchial arteries are the usual source, but a number of case reports detail successful pulmonary arterial embolization [6,10–12]. As yet there are no randomized controlled trials comparing surgical resection to bronchial or pulmonary arterial embolization for hemoptysis. Surgical lobectomy does provide definitive management when the bleeding point can be localized to one lobe and the patient is operable, however, in an intensive care setting, postoperative complications were encountered in 50% of patients [16], with mortality in 20% [17]. As minimally invasive techniques are more desirable to patients and clinicians alike, perhaps it would be difficult to enroll patients in a large randomized trial comparing surgery to embolization in the future.
The advent of contrast-enhanced MDCTA has enabled a noninvasive, first-line method of localizing the site of arterial bleeding in the setting of massive hemoptysis, as eloquently shown in the figures presented here. This MDCTA tool allows for endovascular treatment planning, prior to invasive angiography, to ensure a one-step treatment option for the unstable patient. In this case, initial MDCTA localized the site of bleeding to an aneurysm of the pulmonary artery, which was not expected, as a bronchial arterial source is more common. Without the MDCTA, we would have performed bronchial arterial angiography as the first-line investigation to determine the hemorrhagic source. Remy et al. previously reported that pulmonary angiography must be performed as the second-line investigation with negative bronchial and nonbronchial systemic arteriography [8]. Picard et al. suggested that contrast-enhanced helical CT could be of interest as a first-line investigative tool [11]. More detectors, improved spatial resolution, faster scan times, and better reconstruction algorithms have all contributed to acquiring CT images capable of diagnosing many vascular anomalies. We agree with Picard et al. and currently use MDCTA as the first-line investigation in acute arterial bleeding from all anatomical sites.
Arterial transcatheter embolization can be performed with a number of different commercially available substances, including particulate materials such as embospheres, proximal blocking agents comprising coils, glue, Gelfoam, detachable balloons, stent grafts [18], and, finally, sclerosing agents such as alcohol. Many of the other authors who have successfully embolized Rasmussen’s aneurysms used coils to achieve occlusion of the aneurysm [6, 10–12]. However, there is a rupture rate with coils, which can prove fatal [7]. In this case, Glubran 2 (NBCA-MS co-monomer; GEM) was the chosen embolization agent to occlude the narrow-necked aneurysm. We favored glue in this acute setting, as the time taken for embolization with glue in the setting of hemodynamic instability is considerably less than that for coils [19]. Glubran 2 is a synthetic surgical glue which polymerizes rapidly on contact with blood to form an elastic film of high tensile resistance to enable hemostasis. If the polymerization is too rapid, this can cause the tip of the microcatheter to become glued to the vessel lumen; thus to reduce the polymerization time and to enable radio-opacity, the glue is mixed with Lipiodol (480 mg I/ml [Guerbet]). Histoacryl (n-butyl-cyanoacrylate [NBCA]; B. Braun, Melsungen, Germany), another synthetic surgical glue, can be used as an alternative to Glubran 2 and, again, should be diluted with Lipiodol. However, we have found that Glubran 2 allows more controlled embolization than histoacryl. It appears less sticky and allows more time for embolization before catheter withdrawal.
Rasmussen’s aneurysm is a rare cause of massive hemoptysis which can be life-threatening. This case highlights the importance of MDCTA in localizing the source of arterial hemorrhage in an unstable patient with active bleeding and the ability of interventional radiologists to successfully employ endovascular techniques to control active hemorrhage.
References
Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH (2001) Thoracic sequelae and complications of tuberculosis. Radiographics 21:839–860
Raviglione MC, O’Brien RJ (2005) Tuberculosis. In: Fauci AS, Hauser SL, Longo DL, Jameson L, Kasper DL, Braunwald E (eds) Harrison’s principles of internal medicine, 16th ed. McGraw-Hill, New York, pp 953–966
Kwon W, Kim YJ, Lee YH, Lee WY, Kim MS (2006) The effectiveness of embolotherapy for treatment of haemoptysis in patients with varying severity of Tuberculosis by assessment of chest radiography. Yonsei Med J 47:377–383
Sung YS, Suh KJ, Kim YJ (1992) Bronchial artery embolization: clinical analysis of 129 cases. J Korean Radiol Soc 28:505–512
Kim SM, Kim YJ, Yang HS, Lee MS, Sung KJ (1994) Arterial embolization for management of haemoptysis. J Korean Radiol Soc 30:1029–1034
Santelli ED, Katz DS, Goldschmidt AM, Thomas HA (1994) Embolization of multiple Rasmussen aneurysms as a treatment of haemoptysis. Radiology 193:396–398
Pantankar T, Prasad S, Deshmukh H, Mukherji SK (2000) Fatal haemoptysis caused by ruptured giant Rasmussen’s aneurysm. Am J Roentgenol 174:262–263
Remy J, Lemaitre L, Lafitte JJ, Vilain MO, Saint Michel J, Steenhouwer F (1984) Massive haemoptysis of pulmonary arterial origin: diagnosis and treatment. Am J Roentgenol 143:963–969
Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL (1996) Massive haemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology 200:691–694
Sanyika C, Corr P, Royston D, Blyth DF (1999) Pulmonary angiography and embolization for severe haemoptysis due to cavitary pulmonary tuberculosis. Cardiovasc Interv Radiol 22:457–460
Picard C, Parrot A, Boussaud V, et al. (2003) Massive haemoptysis due to Rasmussen aneurysm: detection with helicoidal CT angiography and successful steel coil embolization. Intens Care Med 29:1837–1839
Jayet PY, Denys A, Zellweger JP, et al. (2004) Successful embolization of Rasmussen’s aneurysm for severe haemoptysis. Swiss Med Wkly 134:705–706
Hamano J, Shiotani S, Yamazaki K, Suzuki M, Ishikawa H (2004) Postmortem computed tomographic (PMCT) demonstration of fatal haemoptysis by pulmonary tuberculosis—radiological-pathological correlation in a case of rupture of Rasmussen’s aneurysm. Rad Med 22:120–122
van den Heuvel MM, van Rensburg JJ (2006) Images in clinical medicine: Rasmussen’s aneurysm. N Engl J Med 19:355:e17
Remy-Jardin M, Wattinne L, Remy J (1991) Transcatheter occlusion of pulmonary arterial circulation and collateral supply: failures, incidents and complications. Radiology 180:699–705
Conlan AA, Hurwitz SS, Krige L, Nicolaou N, Pool R (1983) Massive haemoptysis. Review of 123 cases. J Cardiovasc Surg 85:120–124
McCollun WB, Mattox KL, Guinn GA, Beall AC (1975) Immediate operative treatment for massive haemoptysis. Chest 67:152–155
Chou MC, Liang HL, Pan HB, Yang CF (2006) Percutaneous stent-graft repair of a mycotic pulmonary artery pseudoaneurysm. Cardiovasc Interv Radiol 29:890–892
Wakhloo AK, Perlow A, Linfante I, et al. (2005) Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulas: technical considerations and clinical outcome. AJNR 26:1888–1897
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keeling, A.N., Costello, R. & Lee, M.J. Rasmussen’s Aneurysm: A Forgotten Entity?. Cardiovasc Intervent Radiol 31, 196–200 (2008). https://doi.org/10.1007/s00270-007-9122-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-007-9122-6