Article Text
Statistics from Altmetric.com
Description
A 33-year-old woman presented with acute jaundice, mild epigastric discomfort and lethargy. Despite a marked derangement in her liver function tests, coagulation and acid–base status, extensive investigation into a possible aetiology was futile. An abdominal ultrasound showed a small liver and no ascites. A diagnosis of seronegative subacute hepatitis was made.
The patient fulfilled the King's College criteria for liver transplantation and, soon after, had an uneventful liver transplant from a brain-dead donor. Postoperatively, she was transferred to the intensive care unit. Following extubation, she was managed with ambisome antimicrobial prophylaxis, and with azathioprine, tacrolimus and prednisolone for immunosuppression.
On day 5 postoperatively, she reported new-onset worsening right upper quadrant pain and nausea. There was associated vomiting and intolerance of nasogastric tube feeding. Her abdomen was soft and generally tender. There was little change in her postoperative liver function tests.
A CT of the abdomen and pelvis was performed, which showed an infarcted segment of the right lobe of the liver (figure 1), and compression of the origin of the coeliac axis by the median arcuate ligament (MAL) of the diaphragm (figures 2⇓–4). The patient was taken back to theatre, where this ligament was located and divided. The splenic artery was ligated and divided to further improve hepatic artery flow. Postoperative ultrasound Dopplers of the hepatic vessels confirmed adequate resistive indices and flow. Over the course of the next few days, her abdominal pain improved, and there was an improvement in her liver function tests.
Low-attenuation within segment 7 of the liver, indicative of infarction.
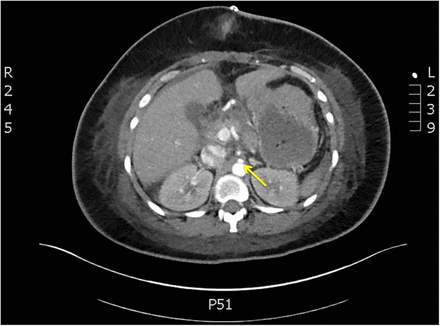
An axial image of the abdomen, which shows the extremely narrowed coeliac axis (arrow) posterior to the median arcuate ligament. The median arcuate ligament is formed by the fusion of the two crura.
A magnified view of figure 2, showing the right and left crura (arrowheads), behind which lies the compressed coeliac artery (arrow).
A lateral projection showing stenosis of the coeliac axis on its superior aspect (arrow) caused by the median arcuate ligament.
Coeliac artery compression syndrome (or Dunbar syndrome) is defined as abdominal pain related to compression of the coeliac artery by the MAL.1 The aetiology is uncertain but believed to be related to both, ischaemic and neuropathic mechanisms.2 This uncertainty was supported by an uneventful liver transplant in this case. A reduction in coeliac artery blood flow can lead to hepatic artery thrombosis and subsequent graft loss.3 Appropriate imaging can allow for early detection and surgical management.
Learning points
Coeliac artery compression syndrome (or Dunbar syndrome) is defined as abdominal pain related to compression of the coeliac artery by fibres of the median arcuate ligament (MAL). The MAL traverses the aorta just superior to the origin of the coeliac artery, and bridges the crura of the diaphragm.
In liver transplantation, the collateral circulation to the liver is compromised and the coeliac artery remains the only source of arterial blood. It is therefore imperative to identify and remove any obstruction of the coeliac axis to prevent hepatic artery thrombosis and subsequent potential graft loss.
The diagnosis of coeliac artery compression is challenging and often one of exclusion. The diagnosis can be confirmed by CT and MRI, or with traditional angiography.
Footnotes
Contributors MA gained consent, gathered the clinical information for the case, was involved in the diagnostic process and assisted with the write up of the case. JP critically reviewed the article for intellectual content, and assisted with the write up of the case. MA and JP were involved with the conception and design of the manuscript, and review of the literature.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.