Article Text
Abstract
The early engraftment phase of allogeneic haematopoietic stem cell transplantation can be associated with a number of oromucosal infective complications. While the routine use of prophylactic acyclovir has reduced the incidence of herpes simplex virus (HSV) reactivation, there is an increasing prevalence of acyclovir resistance within this cohort of patients. The authors present a case of acyclovir-resistant HSV reactivation in a 26-year-old woman 7 days post T-deplete sibling allograft on a background of combined cyclophosphamide and total body irradiation myeloablative conditioning, successfully treated with foscarnet and cidofovir therapy and discuss the differential diagnoses for early/late engraftment oral disease.
- haematology (incl blood transfusion)
- malignant and benign haematology
- dentistry and oral medicine
Statistics from Altmetric.com
Background
Haematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for the management of haematological malignancy, and non-malignant haematological dyscrasias—including myelodysplastic disorders and haemoglobinopathies. From an oral perspective, HSCT can be associated with both acute and chronic complications, either secondary to pre-engraftment conditioning regimens or directly resulting from transplant.1
Acute oral complications are seen in up to 70% of patients receiving HSCT and include oral mucositis, graft-versus-host disease (GvHD) and infection. The incidence of oral mucositis is in the region of 73%–86%,2 with the severity of its presentation directly correlated to the intensity of the conditioning regimen implemented. The incidence of severe mucositis (WHO Oral Mucositis Grading Scale II–IV) is higher in patients receiving total body irradiation (TBI),3 however, other determinants such as the use of methotrexate in GvHD prophylaxis have also been identified.4
GvHD is a common complication of HSCT and remains a leading cause of non-relapse post-transplant mortality. Acute GvHD is reported in up to 50% of HSCT recipients with human leucocyte antigen (HLA) mismatch, a well-documented risk factor for its development.5
Post-HSCT infection is the leading cause of post-transplant morbidity in the pre-engraftment, immediate and late postengraftment periods, with a number of transplant factors influencing immune reconstitution—HLA disparity, T cell depletion, GvHD.6 Pre-engraftment is characterised by aplasia, with recovery of neutrophil counts by week 2–3. The postengraftment immunodeficient state is secondary to reduced number of natural killer cells of the innate immune system and T cells of the adaptive immune system, which can take up to 24 months to normalise.7
The combination of neutropenia and disrupted mucosal barrier protection, secondary to mucositis, in the early pre-engraftment period is associated with a high risk of HSV infection. Reactivation was seen in up to 80% of HSV-seropositive HSCT recipients prior to the introduction of routine antiviral prophylaxis. Routine prophylactic use of acyclovir following allogeneic HSCT has reduced the incidence of HSV reactivation to 0%–3%.8 The recent emergence of acyclovir-resistant HSV, particularly among immunocompromised patients, poses a challenge in the acute management of HSCT patients postengraftment.
Case presentation
A 26-year-old woman diagnosed with myelodysplastic syndrome with multi-lineage dysplasia received a T-deplete sibling allograft on a background of combined cyclophosphamide and TBI myeloablative conditioning. She was referred to the oral medicine department secondary to the onset of oral ulceration which developed day 7 postengraftment. As per protocol, the patient was receiving prophylactic oral acyclovir (200 mg, three times a day) at this stage.
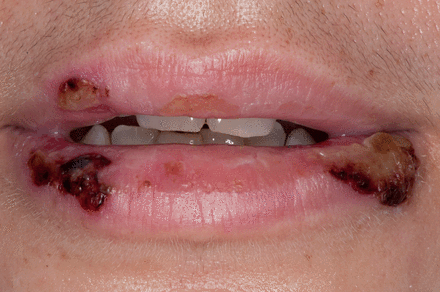
Examination revealed diffuse irregular ulceration of the keratinised and non-keratinised oromucosae, with no dermatomal confinement. Extensive pseudomembranous cover was noted, with no diffuse mucosal atrophy or suggestion of underlying lichenoid phenotype. There was no noted oropharyngeal involvement, or haemorrhagic crusting to the lip prolabia pathognomonic of erythema multiforme. A single concentric crusted lesion was noted in the mental region (figures 1–4). In terms of wider mucocutaneous involvement, there was a large blister of the left dorsal foot and an acneiform rash of the back and shoulder.
Pretreatment perioral HSV presentation.
Bilateral erosive disease of the hard palate.
Fibrinous pseudomembrane of retromolar trigone.
Superficial erosion of the attached mandibular gingivae.
Investigations
PCR swab testing of the oral ulceration was positive for HSV-1 and excluded Epstein-Barr Virus, Varicella Zoster Virus and Cytomegalovirus.
HSV-1 drug resistance testing was carried out and demonstrated HSV-1 UL23 mutation, associated with acyclovir resistance.
Differential diagnosis
Differential diagnosis at initial presentation included erythema multiforme secondary to HSV-1, paraneoplastic autoimmune multiorgan syndrome (PAMS) and HSV-1 recrudescence.
Viral reactivation occurs frequently following HSCT and given the extensive oral ulceration, crusting of the lips and concentric lesion of the chin, erythema multiforme secondary to HSV-1 reactivation was the first diagnosis considered. The lack of classical cutaneous ‘target’ lesions and the failure of the oral symptoms to self-resolve made this diagnosis less likely.
In combination with the history of blistering of the left dorsal foot, the suspicion of PAMS was raised following the failure of improvement seen with initial treatment dose intravenous antiviral therapy. A close haematology–oncology review was carried out and confirmed successful graft, negating this possible diagnosis.
The final diagnosis reached was HSV-1 recrudescence. This was initially considered somewhat unlikely given both the severe presentation and concomitant antiviral prophylaxis. In view of this differential, HSV-1 drug resistance testing was carried out and demonstrated HSV-1 UL23 mutation, associated with acyclovir resistance.
Treatment
Prior to HSV-1 drug resistance testing, treatment was escalated to intravenous treatment dose acyclovir (10 mg/kg three times a day) for 10 days, with partial clinical response (figures 5–7). Subsequent switch to oral valacyclovir (500 mg two times per day) resulted in rebound disease activity.
Evident healing of vermillion ulceration following escalation of intravenous acyclovir to treatment dose.
Limited intra-oral clinical improvement following escalation of intravenous acyclovir to treatment dose.
Limited intra-oral clinical improvement following escalation of intravenous acyclovir to treatment dose.
The limited disease modifying action of acyclovir and analogues was later attributed to HSV-1 UL23 mutation acyclovir resistance. Cross-resistance was suggested by the limited improvement with foscarnet therapy (90 mg/kg three times a day). Substitution for intravenous cidofovir (375 mg) once weekly for 4 weeks resulted in complete remission of oral ulceration (figures 8–10). The patient was then returned to oral acyclovir (400 mg three times a day) as per routine prophylaxis guidelines.
Perioral healing secondary to 3-week course of intravenous cidofovir (5 mg/kg, once weekly).
Clinical appearance of hard palate post-3-week course of intravenous cidofovir (5 mg/kg, once weekly).
Clinical appearance of retromolar trigone secondary to 3-week course of intravenous cidofovir (5 mg/kg, once weekly).
Outcome and follow-up
At 3-month review, there was no evidence of mucosal activity, with stable oromucosal appearances.
Discussion
The antiviral action of acyclovir results from its selective phosphorylation by the viral enzyme, thymidine kinase. The resultant triphosphate metabolite is a competitive inhibitor for viral DNA polymerase, resulting in viral inactivation and DNA chain termination.9 Resistance derives from mutations to either DNA polymerase or the thymidine kinase enzyme—with three distinct phenotypes identified in terms of TK activity—absent, reduced or altered.10 Unlike acyclovir, cidofovir and foscarnet do not require enzymatic phosphorylation and act as direct inhibitors/alternative substrates for HSV DNA polymerase—thus are effective second-line agents in treating acyclovir-resistant isolates.
Literature review demonstrates a prevalence of acyclovir resistance of 14%–46.5% within HSCT recipients with clinically confirmed HSV infection,11–13—which is markedly higher than in immunocompetent patients. Concomitant GvHD is reported to be an additional significant risk factor for the development of antiviral resistance.14
This case report demonstrates successful treatment of HSV resistance with both cidofovir and foscarnet, however, there are case reports of cross-resistance to acyclovir and foscarnet.15 Escalation to cidofovir in this isolated example has proven clinically effective.16 Both medications show a similar adverse side effect profile in terms of nephrotoxicity.
Risk factors for infection postallograft are multifactorial—relating to impaired innate and acquired immune responses. These include the underlying host disease, type of conditioning regime implemented, the type of graft and presence/absence of GvHD.17 In this case, infection occurred in the early engraftment phase (<30 days), with evident neutropenia and disruption of mucosal barriers underlying susceptibility. Both acute leukaemia and myelodysplastic syndrome as underlying host diseases result in profound preallograft neutropenia and risk factors for infection. The myeloablative conditioning regimens used in this case, with cytotoxic chemotherapy and TBI, result in mucositis and neutropenia which further exacerbate the risk of infection over reduced intensity regimens.18 HLA matching is also a contributory factor to the development of GvHD and risk of superinfection.
Learning points
An understanding of conditioning regimens and the timeline of immune reconstitution can aid clinicians in identifying likely causes of acute and/or chronic complications in haematopoietic stem cell transplantation (HSCT) patients during these distinct clinical phases.
Despite routine antiviral prophylaxis regimens post-HSCT, viral recrudescence should be considered in cases of oromucosal ulceration due to increasing resistance.
Prodromal phenomena, irregular lesion borders and vesicular phenotype are often diagnostic features of viral ulceration. Direct methods for specimen collection of suspected herpes simplex virus lesions include de-roofing of vesicles and use of a Rayon swab, rotated firmly of the lesion base. For older lesions, the Tzanck test can be used for diagnosis, by scraping the base of the lesion with the edge of a scalpel blade.
Ethics statements
Patient consent for publication
Acknowledgments
UCLH Haematology Department, Dr Ben Carpenter and Dr Arian Laurence, for their care of the study patients.
References
Footnotes
Contributors TS is responsible for the overall content of this report as guarantor—accepting full responsibility for the finished work and/or the conduct of the study, access to the data, and control of the decision to publish. MH and DI have been instrumental in the planning, conduct and reporting of the work described in this article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.