Article Text
Abstract
Gastrointestinal stromal tumour (GIST) is a recent recognised tumour entity. In the past, those tumours were classified as leiomyomas, leiomyosarcomas and leiomyoblastomas, but it is now evident that GIST is a separate tumour entity and is the most common sarcoma of the gastrointestinal tract especially with advances in immunohistochemical staining techniques and improvements in microscopic structural imaging. We present a case of GIST of unusual location and presentation pattern, with an overview over current GISTs’ diagnosis and management strategies. The precise incidence and tumour behaviour of rare extragastrointestinal stromal tumour (EGIST) remain to be clarified. Further research is needed in large series with long duration of follow-up and modified risk stratification assessment tailored for EGISTs.
- oncology
- surgery
- general surgery
- surgical oncology
- radiology (diagnostics)
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
Gastrointestinal stromal tumour (GIST), a rare recognised entity until 2000, the era that has marked the discovery of an activating mutation of the c-kit tyrosine kinase found in almost all GIST tumours and the ability to target those mutations with the specific tyrosine kinase inhibitor (imatinib mesylate).1 2
GIST tumours now represent approximately about 3%–5% of all soft tissue sarcomas, respectively 3% of gastrointestinal (GI) tumours.2 Most GISTs arise from the stomach (50%–70%) and small intestine (20%–30%), including the duodenum, jejunum and ileum. Other locations are the large intestine (5%), and the oesophagus in 2%–5% of cases.2 3 Small numbers of extragastrointestinal stromal tumours (EGISTs) have been reported in the literature; most of those were case reports or cohort analysis (Mettinen et al, analysis of 112 cases) where clinicopathologic correlation and long-term follow-up data of such tumours are scant.4 Other groups (FanFing and colleagues, analysis of 114 mesenteric GIST) have reported EGISTs in other rare sites such as in mesenteric location.5
Case presentation
A 67-year-old male patient referred from urology service where he was following for benign prostatic hyperplasia (BPH), when an incidental finding of a right iliac fossa (RIF) mass was noted on ultrasound (US) study. The patient reported to have a 1-year history of progressive growing of a painless abdominal mass; he denied any abnormal bowel habits or any other symptoms. The patient’s relevant history included diabetes mellitus (DM), hypertension (HTN), mild renal impairment. Of notice, he underwent resection of a small bowel tumour 20 years ago in another facility when he presented with upper GI bleeding and shock. At that time, a bleeding jejunal tumour was resected and the final histopathology reported smooth muscle tumour of undetermined malignant potential (SMTUMP). No further treatment was given afterwards and the patient reported living a normal life. On physical examination, a large solid, ill-defined non-tender mass was found, measuring around 13 cm in diameter, occupying the RIF and the lower abdomen. Completed work up resulted in unremarkable colonoscopy and tumour markers (CEA, CA 19.9, AFP).
Investigations
Requested abdominal US (figure 1), showed a well-defined heterogeneous slightly echogenic solid mass with bull’s eye appearance and a central necrotic geographic area likely representing a central ulceration. It showed no significant flow on colour Doppler which is typical for large GISTs.6 The mass measured 11.3×12.7×14 cm3. Mural nodules were noticed, likely originating from the mesentery or the retroperitoneum.
(A) Transverse grey scale ultrasound (US). (B) Colour Doppler US.
A non-enhanced CT scan of the abdomen and pelvis with oral contrast (figure 2) revealed a large low attenuated right midline pelviabdominal extraintestinal mass with left dense lobule or mural nodule (arrow). The origin of the mass was likely from the mesentery or the retroperitoneum anterior to the aortic bifurcation. The lesion measured 17.6×17.6×11 cm3. It had no connection to adjacent bowel or organs.
Coronal non-enhanced abdominal CT scan.
MRI of abdomen and the pelvis (figure 3) showed a necrotic mass originating from the mesentery or the retroperitoneum containing internal debris, ulceration and septations.
(A) MRI abdomen coronal T2 fat saturation. (B–D) Coronal and sagittal T2 MRI. (E, F) Sagittal diffusion and ADC map MRI sequences. (G, H) Axial and sagittal T1 fat saturation post gadolinium contrast administration MRI shows the progressive enhancement of the wall and mural nodule (arrows).
The solid component in the mass showed hypointense signal in T2 WIs (A–D), which was more prominent on the left side with a nodule likely representing fibrous tissues. It appeared as well hypovascular (G) with progressive enhancement in the delayed sequences (H), and showed small areas of restrictive diffusion in the left mural nodule (E and F, white arrows). The cystic components showed T2 shine through effect.
Treatment
The patient underwent laparotomy exploration, where a giant whitish well encapsulated solid mass was found as shown in figure 4, measuring around 15 cm in diameter. The mass was not invading nearby organs or structures . It had a posterior attachment to the pelvic retroperitoneum (figure 5), from which it was separated completely. Completed exploration to the peritoneal cavity showed no other abnormality. The intraoperative picture suggested an extragastrointestinal retroperitoneal tumour.
Whitish well encapsulated tumour.
The mass had no attachment to nearby structures; it had only a posterior attachment to the retroperitoneum shown in the picture.
Differential diagnosis
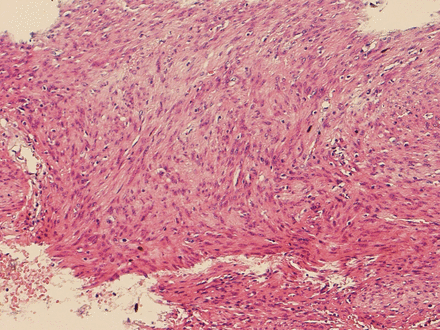
Pathological examination of the tumour reported a 15 cm, cystic well encapsulated tumour with a thickened wall of 12 mm. On cross section, the tumour had a fleshy granularity with multiple pale areas of necrosis and reddish regions of haemorrhage which was suggestive of GIST.4 Microscopic examination showed marked haemorrhagic and cystic degeneration. Spindle cells were having cigar shaped nuclei and pale eosinophilic cytoplasm with indistinct membrane. In between, chronic inflammatory cells composed of lymphocytes were seen. No atypia was present, minimal mitosis 2/50 high power fields (HPFs) was noted and margin was free of tumour cells (figure 6). Immunohistochemistry (IHC) stained negative for actin and β-catenin in spindle cells and CD117 stained positive (figure 7). Given the above-mentioned characteristic of the tumour, tumour cells and a positive stain of CD117, the overall picture suggested a retroperitoneal EGIST.
Microscopic histopathology showing streaming bundles of bland looking delicate spindle cells with faint eosinophilic cytoplasm and inconspicuous nucleoli, overt perinuclear clearing (halos) and no brisk mitotic activity.
Immunohistochemistry showing expression of CD117 marker in tumour cells.
Outcome and follow-up
At the time of this report, the patient is 5-month post-operation. He was referred to oncology service for adjuvant treatment, which was considered due to large tumour size and his history favouring a theory of recurrent GIST. A hypothesis was proposed that his resected small bowel tumour 20 years ago which was diagnosed as SMTUMP at that time, when IHC analysis methods were not available and before the evolution of imatinib, was a primary GIST tumour with a slow malignant potential, and that his current presentation of an extraintestinal tumour mass is a latent recurrence rather than a primary EGIST. He is now receiving GLEEVEC (imatinib mesylate) tablets 400 mg per day for an estimated duration of 3 years . His recent CT scan of abdomen, pelvis and chest during follow-up showed no evidence of tumour recurrence (figure 8).
Non-enhanced 6-month follow-up CT scan shows no local recurrence.
Discussion
GIST’s particular clinical features, histological and uncommon molecular characteristics, have led many to consider separating it from the usual smooth muscle tumours into a specific entity.1 2 A striking, uniform immunoreactivity for kit receptor (CD 117) marked such stromal tumour, which was abscent in other types smooth muscle, Schwannian tumours of the GI tract and other sarcomas of the GI tract.7 GISTs show morphological and immunophenotypic similarities with intestinal cell of cajal, a pacemaker system in the GI tracts.2 4
Differential diagnosis of GISTs has been very much facilitated by IHC test, using a complete and specific panel of antibodies for mesenchymal tumours. Most GISTs (>90%) show overexpression of the receptor tyrosine kinase KIT (CD117) by IHC, a proportion of GISTs (5%) which are CD117-negative exists.2 3
Approximately 70% of GISTs express CD34 and may express smooth muscle actin on IHC.1 7 Other commonly used markers include caldesmon, S-100 (a neural cell marker) protein and keratin, which can be variably immunoreactive in GISTs.8 Differential diagnosis includes tumours with nervous differentiation: gastric schwannomas (intensely positive for S100-protein, but negative for CD117), tumours with fibrous differentiation such as intra-abdominal fibromatosis, which affects the stomach (CD117-negative), inflammatory gastric polyp and inflammatory myofibroblastic pseudo-tumour (both negative for CD117 and CD34). IHC can also exclude the retroperitoneal undifferentiated liposarcoma, and the other two mesenchymal tumours positive for c-kit: metastases of malignant melanoma (HMB45 and Melan A-positive) and angiosarcoma (CD31-positive).9
GIST tumours are well defined, not encapsulated, firm in consistency and whitish in colour. Small lesions have a homogeneous aspect on overall section surface, while large lesions may present with zones of necrosis, haemorrhage and cystic degeneration. Grossly, they appear fleshy pink or tan-white cut surface with haemorrhagic foci. Microscopically, they can have a moderate or high cellularity, and can be divided into spindle cell, epithelioid, signet ring cell, pleomorphic, oncocytic variants or those with myxoid stroma variant.2 8 9
Small size GISTs can be discovered incidentally during imaging or endoscopy. Symptoms vary depending on the location and size of the tumour, those related to the tumour mass effect (abdominal pain, discomfort, distension and a palpable mass), or others presenting with anaemia and GI haemorrhage.10 11
Contrast-enhanced abdominal and pelvic CT scan is the investigation of choice for staging and follow-up. MRI may be an alternative such as for rectal GISTs. Chest CT scan and routine laboratory testing complement the staging work up. Evaluation of fluorodeoxyglucose (FDG) uptake using an FDG-positron emission tomography (PET) scan, or FDG-PET-CT/MRI, is useful for early detection of tumour response to molecular-targeted therapy or neoadjuvant therapy.11 12
On imaging, the ‘Embedded organ’ sign is a useful and valid tool for identifying the organ of origin.13 GIST usually shows intense homogeneous enhancement; however larger lesions show necrosis and appear heterogeneous and hypovascular.14–16 The EGISTs still have no typical radiological features and they have a non-specific appearance with wide deferential diagnosis including leiomyosarcoma, fibrosarcoma, liposarcoma, solitary fibrous tumour, paragnglioma, schwannoma and lymphoma. EGIST presenting as a mass in the omentum, peritoneum or retroperitoneum could be a primary GIST or a metastatic one.17
Metastases in GISTs have been reported in 50% of the patients. The liver is the organ with the most frequent metastases (65%), followed by the peritoneum (21%). Metastases to lymph nodes, lungs and bones are considered rare sites.2 11 The question in our case is whether it is a primary EGIST or a latent recurrence from a previous small intestinal GIST diagnosed in the past as SMTUMP.
Noted that small (<1 cm) incidentally found GISTs behave almost invariably in a benign fashion, while tumours arising from the small bowel, colon, rectum or mesentery are generally associated with less favourable outcome than those arising from the stomach.11 Rare GISTs, referred as EGISTs, that arise in the abdominal cavity outside of the GI tract were associated with the most unfavourable outcome, while some EGISTs might have been metastasised from an undetected primary tumours.18
Estimating the risk of recurrence is an important factor as many GISTs have an uncertain malignant potential. Adjuvant therapy has become a standard praxis in the management of such tumours, and it can be used as a guide for risk of recurrence.2 10 Several risk stratification factors have been proposed (table 1): the National Institute of Health (NIH) consensus criteria, the modified NIH consensus criteria and the Armed Forces Institute of Pathology criteria. Risk factors incorporated included; tumour size and site, mitotic count per HPFs and tumour rupture. 2 12 18–20 The TNM classification for staging has several limitations and its use is not recommended in this disease.10
Stratification schemes for GIST recurrence used in some studies. (Adopted from Joensuu, Heikki, et al. ‘risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts.’ The Lancet Oncology, vol. 13, no. 3, 2012, PP. 265–274, doi:10.1016/s1470-2045(11)70299-6.)
Mutational analysis can play a rule in GIST, as mutations involving KIT and PDGFRA can confirm the diagnosis of GIST if doubtful and in rare cases of CD117/DOG1-negative suspected GIST, which are of 5% incidence. It has as well a predictive value for the sensitivity to molecular-targeted therapy and a prognostic rule, since some GISTs are insensitive to the drug imatinib in cases of PDGFRA exon 18 mutation D842V.11 Some consensus suggested that mutational analysis inclusion in the diagnostic work up of GISTs should be considered a standard practice.12
Surgery is the standard treatment for localised GISTs. The tumour should be removed en-bloc with its pseudocapsule to yield an adequate resection margin. The optimal width of the tumour-free margin has not been defined. Regional lymph node resection is of unproven value, since GISTs rarely metastasise to lymph nodes. Tumour rupture is associated with poorer outcomes and should be reported, as it carries a risk of tumour seeding.11 12
The presence of metastasis does not contraindicate surgery of the primary tumour, fwhile neoadjuvant imatinib should be considered for those large gastric or rectal primaries where immediate resection is likely to be morbid, for example total gastrectomy or abdomino-perineal resection. In such situations, mutational analysis is mandatory prior to the initiation of imatinib therapy.11
The introduction of adjuvant therapy (imatinib) has revolutionised the management of primary GISTs and should be considered a standard treatment in all patients with significant risk of recurrence following resection of primary GISTs.21 The standard therapy for imatinib is 400 mg daily for 3 years, and randomised clinical studies are ongoing to test longer durations of adjuvant therapy.2 12 21 Patients who progress despite imatinib dose escalation or are intolerant to imatinib are candidates for dose escalation or a trial of other tyrosine kinase inhibitors such as sunitinib, a standard second line treatment.11
There are no published data to indicate the optimal routine follow-up policy of surgically treated tumours with localised disease. Such is the case with our patient with a suspect of recurrence 20 years after primary surgical excision before the imatinib era. Nowadays, it is recommended to do annual enhanced CT scan of the abdomen and pelvis for 5 years in low-risk patients after the surgery, and every 6 months for high-risk patients and those on adjuvant imatinib therapy. After that every 3–4 months for the first 2 years and every 6–12 months for 10 years from stopping imatinib.16 Relapses occur more often to the liver and peritoneum, and rarely to lung and bone. Risk assessment based on the mitotic count, tumour size and tumour site may be useful in choosing the routine follow-up policy. High-risk patients generally relapse within 1–3 years from the end of adjuvant therapy. Low-risk patients may relapse later, given that the disease is likely to be growing slowly.14
Learning points
The unusual occurrence of CD117-positive tumours outside the gastrointestinal tract questions the origin of such tumours from the interstitial cells of cajal and whether the parameters used for predicting the prognosis of GIST are suitable for EGISTs’ evaluation.
The origin, incidence and tumour behaviour of EGIST are a subject still to be investigated.
Further research is needed in large series with long duration of follow-up to set up a tailored diagnosis and management guidelines for such type of tumours.
References
Footnotes
Contributors AAA has made substantial contributions to the conception, design of the work, the acquisition, analysis, interpretation of data, have drafted the work and revised it. She has approved the submitted form and agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved and the resolution documented in the literature. GB has contributed to the conception, design of the work, the acquisition, analysis, has revised the work and approved the submitted form.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.