Article Text
Summary
Calcium has diverse roles in neuromuscular mechanisms. Within the cardiovascular system, hypocalcaemia is known to both impair myocardial contractility and prolong the QT interval, predisposing to ventricular arrhythmias. We present a case of chronic hypocalcaemia with prolonged QT interval associated with an atrial arrhythmia. Recent studies of congenital long QT syndrome suggest there is also a predisposition to atrial arrhythmias. Our case raises the hypothesis that acquired causes of long QT cause similar repolarisation abnormalities that predispose to atrial arrhythmias.
Statistics from Altmetric.com
Background
This case introduces the concept of ‘atrial torsades de pointes’ as well as providing a reminder of the late complications of thyroid surgery.
Case presentation
A 34-year-old woman presented with a 5 day history of palpitations associated with dyspnoea. She reported palpitations occurring up to 20 times a day, with episodes lasting between 5–30 min. There had been no syncopal episodes or collapse. Caffeine use was minimal and no other stimulants were being taken. There was no history of alcohol excess. No regular medication was taken. She had undergone partial thyroidectomy in the Philippines, aged 11 years, for a multinodular goitre. On examination, a thyroidectomy scar was noted and pronounced dystrophy of the nails was present (fig 1). Carpopedal spasm could be induced using a sphygmomanometer, and Chovstek’s sign was present. The resting pulse was 98 beats/min and blood pressure was 129/81 mm Hg.
Nail dystrophy due to hypocalcaemia.
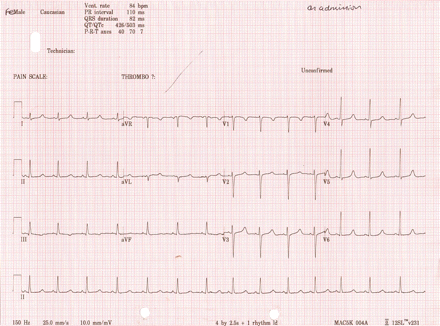
An electrocardiogram (ECG) (fig 2) while in sinus rhythm demonstrated a corrected QT interval (QTc) of 503 ms (normal range (NR) for women <450 ms). An outpatient 24 h Holter monitor was reported as showing frequent runs of uncontrolled atrial flutter, with rates up to 202 beats/min. The patient was symptomatic, with dyspnoea and palpitations, during these episodes. Subsequent ECGs and telemetry as an inpatient showed uncontrolled atrial fibrillation (AF) or atrial tachyarrhythmia (fig 3). Several electrophysiology specialists reviewed the data and felt AF was the predominant arrhythmia present, but other atrial arrhythmias could not be excluded. No persistent organised atrial activity was noted to suggest ongoing atrial flutter. Some short bursts of apparently organised atrial activity were occasionally seen, but it rapidly degenerated into an irregularly irregular rhythm suggestive of AF. An echocardiogram showed a left ventricular end diastolic dimension of 54 mm (NR 39–53 mm for women). The left ventricle appeared mildly hypokinetic for a young woman.
Electrocardiogram (ECG) showing a corrected QT interval (QTc) of 503 ms while in sinus rhythm.
Image of three ECGs demonstrating an atrial arrhythmia most likely to be atrial fibrillation.
Blood biochemistry revealed Na+ to be 142 mmol/l, K+ 4.3 mmol/l, urea 6.2 mmol/l, and creatinine 61 mmol/l. Corrected calcium was 1.40 mmol/l (NR 2.2–2.6) and albumin was normal at 40 g/l. Magnesium was 0.91 mmol/l (NR 0.7–1.0) while organic phosphate was 2.02 mmol/l (NR 0.8–1.4). Endocrine assessment showed a parathyroid hormone (PTH) value of 1.40 pmol/l (NR 1.6–9.3), free thyroxine (T4) of 10.80 mmol/l (NR 7.5–21.1), and thyroid stimulating hormone (TSH) of 2.81 mmol/l (NR 0.34–5.6).
A diagnosis of surgical hypoparathyroidism was made. The patient was reviewed by the endocrinology service and given intravenous calcium replacement (calcium gluconate 10 mg) at intervals and intravenous magnesium supplementation. Oral calcium replacement and 1-α-calcidol were subsequently started. Long acting cardioselective β-blockers were used to help control the atrial arrhythmia and aspirin instigated for stroke prophylaxis. The QTc was monitored following oral calcium replacement therapy (fig 4). Once the calcium concentration was restored to the normal range, atrial arrhythmias resolved and only sinus rhythm was recorded on Holter monitoring.
Graph showing improvement in the QTc over time as corrected calcium concentrations improved with oral calcium replacement and 1-α-calcidol; the blue legends show the corrected calcium concentration at each QTc.
Discussion
While the majority of calcium in the body is within the skeletal system or bound to albumin in plasma, free calcium is tightly controlled by homeostasis effected by parathyroid hormone (PTH). Transmembrane gradients in calcium concentration drive membrane excitability in muscles, neurones and myocytes.1 Myocardial contraction is dependent on extracellular calcium because the myocardial sarcoplasmic reticulum cannot store sufficient quantities. As such, prolonged and significant hypocalcaemia can result in heart failure.
Among many causes of hypocalcaemia, thyroid surgery, with the inherent risk of parathyroid removal or injury, is a recognised cause of hypoparathyroidism and subsequent hypocalcaemia.2 Patients should undergo long term follow-up to ensure this complication does not present at a later date. Here, it presented 23 years after the initial surgery.
Acute cardiomyopathies have been induced by hypocalcaemia, and calcium replacement and vitamin D supplementation have been shown to reverse the heart failure.3 Furthermore, renal excretion of sodium is partially dependent on intracellular calcium concentrations, and hypocalcaemia may encourage salt and water retention, exacerbating heart failure.3 In our patient, a mild left ventricular hypokinesia was noted with chamber dilatation. However, clinical signs of heart failure did not occur and the ejection fraction remained normal.
Hypocalcaemia is a recognised cause of QT prolongation via prolongation of the plateau phase of the cardiac action potential.4,5 This causes calcium ion channels to remain open for a longer period, allowing a late calcium inflow and the formation of early after-depolarisations.6,7 If threshold for depolarisation is reached, new action potentials are induced, initiating a tachycardia and re-entry. Ventricular arrhythmias can follow, in particular torsades de pointes (TdP; polymorphic ventricular tachycardia) and ventricular fibrillation (VF).8 Therefore, ventricular arrhythmias are a known complication of hypocalcaemia and patients can present with exertional syncope representing TdP and loss of cardiac output.7–9
In contrast, hypercalcaemia and calcium infusions have been shown to reduce immediately the QTc with shortening of the ST segment but prolongation of the T wave; ventricular ectopics are suppressed but heart block and bradyarrhythmias can occur.1,4,5
Recent findings suggest that the disordered repolarisation characteristic of subjects with congenital long QT syndrome (LQTS) occurs in the atria as well as the ventricles.10 Electrophysiological studies showed that congenital LQTS patients had altered atrial electrical conduction such that atrial tachyarrhythmias, such as AF, were more easily induced and persisted for longer than in normal or other patients with AF.10 These induced episodes of AF often had a polymorphic undulating appearance, similar to TdP, and have led to the new term of ‘atrial torsades de pointes’.10 Two large studies have shown that early onset of atrial arrhythmias (typically AF) is 10-fold higher in those with genetically proven LQTS than the general population or matched controls.11 This coincides with genetic studies that suggest the genes that predispose to LQTS (KCNQ1, KCNH2 and KCNA5 encoded potassium channels) also predispose to atrial arrhythmia in the general population.11
These new developments raise the possibility that long QTc triggered by acquired causes, as presented here, could create the same electrophysiological state present in congenital LQTS which destabilises atrial rhythms to trigger atrial arrhythmia. It is possible that hypocalcaemia prolongs atrial repolarisation and therefore triggers atrial arrhythmias with ‘atrial torsades de pointes’ being the underlying abnormality. This mechanism may explain the occurrence of atrial arrhythmia in our patient, though the possibility of a genetic LQTS has not formally been excluded. Furthermore, formal electrophysiological testing may be required because a 12 lead ECG is unlikely to visualise ‘atrial torsades de pointes’, with most previous reports relying on electrophysiology studies or atrial traces from automated defibrillators.
Learning points
-
Hypocalcaemia is a cause of QTc prolongation and this predisposes to ventricular arrhythmias.
-
Atrial arrhythmias have an increased incidence in those with congenital long QTc syndrome. While a genetic basis may be involved, acquired causes of QTc prolongation may similarly predispose to atrial arrhythmias.
-
Hypocalcaemia due to hypoparathyroidism is a known late complication of thyroid surgery. In our case, it presented 23 years after initial surgery.
REFERENCES
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.